324831
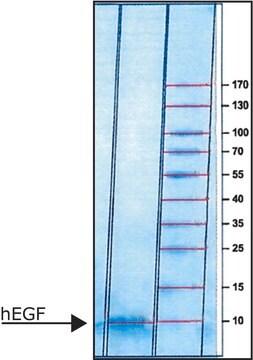
EGF human
≥95% (SDS-PAGE), recombinant, expressed in E. coli, solid, suitable for cell culture
Synonym(s):
EGF, Human, Recombinant, E. coli, Epidermal Growth Factor, Human, Recombinant, E. coli
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Product Name
EGF, Human, Recombinant, E. coli,
recombinant
expressed in E. coli
Quality Level
description
Merck USA index - 14, 3527
assay
≥95% (SDS-PAGE)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
impurities
<1.0 EU/μg Endotoxin (EU/μg EGF)
shipped in
ambient
storage temp.
−20°C
General description
Recombinant, human epidermal growth factor (54 amino acids) expressed in E. coli. Identical to native human EGF except for an additional N-terminal methionine. Human EGF is 70% homologous to mouse EGF and has been shown to have similar biological activity. Mitogenic for a wide range of ectoderm- and endoderm-derived cells from tissue such as breast, cornea, epidermis, dermis, liver, pancreas, nerve, amnion, and adrenal medulla. Binding of EGF to its 170 kDa transmembrane receptor results in dimerization of the receptor, activation of the intrinsic receptor tyrosine kinase, and initiation of the complex signal transduction cascade that leads to cell proliferation and/or differentiation. Acts as a survival factor in preventing apoptosis.
Biochem/physiol Actions
ED₅₀ = 100-400 pg/ml as measured by its ability to stimulate the ³H-thymidine incorporation in an EGF-responsive mouse fibroblast cell line, Balb/3T3.
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C or -70°C) for long term storage or refrigerate (4°C) for short-term storage. Avoid freeze/thaw cycles of solutions. Stock solutions are stable for up to 1 month at 4°C or for up to 3 months at -20°C or -70°C.
Reconstitute to a concentration of ≥10 µg/ml in 10 mM acetic acid containing ≥0.1% HSA or BSA.
Other Notes
Merlo, G.R., et al. 1995. J. Cell Biol. 128, 1185.
Yoshida, T., et al. 1993. Brain Res. Dev.76, 147.
Rubin, J.S., et al. 1991. Proc. Natl. Acad. Sci. USA88, 415.
Carpenter, G., and Wahl, M.I. 1990. In The Epidermal Growth Factor Family in Peptide Growth Factors and Their Receptors (Sporn, M.B., and Roberts, A.B., eds.) Springer-Verlag, New York, p 69.
Downward, J., et al. 1984. Nature311, 483.
Gray, A., et al. 1983. Nature303, 722.
Scott, J., et al. 1983. Science221, 236.
Cohen, S. 1960. Proc. Natl. Acad. Sci. USA46, 302.
Yoshida, T., et al. 1993. Brain Res. Dev.76, 147.
Rubin, J.S., et al. 1991. Proc. Natl. Acad. Sci. USA88, 415.
Carpenter, G., and Wahl, M.I. 1990. In The Epidermal Growth Factor Family in Peptide Growth Factors and Their Receptors (Sporn, M.B., and Roberts, A.B., eds.) Springer-Verlag, New York, p 69.
Downward, J., et al. 1984. Nature311, 483.
Gray, A., et al. 1983. Nature303, 722.
Scott, J., et al. 1983. Science221, 236.
Cohen, S. 1960. Proc. Natl. Acad. Sci. USA46, 302.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Katarzyna A Ludwik et al.
Cell reports, 32(3), 107931-107931 (2020-07-23)
In response to estrogens, estrogen receptor alpha (ERα), a critical regulator of homeostasis, is degraded through the 26S proteasome. However, despite the continued presence of estrogen before menopause, ERα protein levels are maintained. We discovered that ERK1/2-RSK2 activity oscillates during
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service