推薦產品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 1095
traceable to Ph. Eur. Y0000789
traceable to USP 1270377
API 家族
fexofenadine
CofA
current certificate can be downloaded
包裝
pkg of 1 g
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
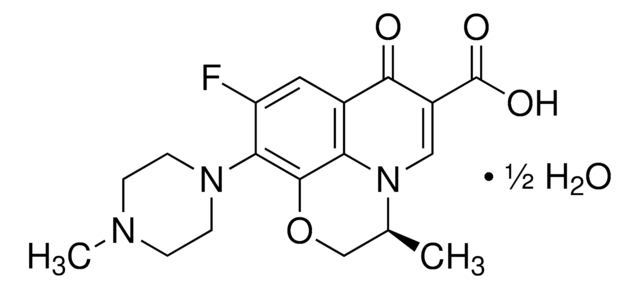
SMILES 字串
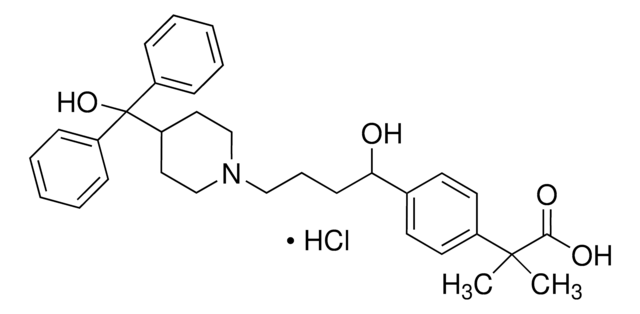
Cl[H].CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN2CCC(CC2)C(O)(c3ccccc3)c4ccccc4
InChI
1S/C32H39NO4.ClH/c1-31(2,30(35)36)25-17-15-24(16-18-25)29(34)14-9-21-33-22-19-28(20-23-33)32(37,26-10-5-3-6-11-26)27-12-7-4-8-13-27;/h3-8,10-13,15-18,28-29,34,37H,9,14,19-23H2,1-2H3,(H,35,36);1H
InChI 密鑰
RRJFVPUCXDGFJB-UHFFFAOYSA-N
基因資訊
human ... HRH1(3269)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
Fexofenadine HCl belongs to the class of drugs known as antihistamines. It acts by selective blockade of H1-receptors and is generally used in the treatment of seasonal allergic rhinitis.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Fexofenadine HCl may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulation by spectrophotometry and high performance liquid chromatography.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生化/生理作用
非索非那定是一种非镇静性 H1 组胺受体拮抗剂。
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAB9160 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Development and validation of a rapid RP-HPLC method for the determination of cetirizine or fexofenadine with pseudoephedrine in binary pharmaceutical dosage forms
Karakus S, et al.
Journal of Pharmaceutical and Biomedical Analysis, 46(2), 295-302 (2008)
Spectrophotometric and high performance liquid chromatographic determination of fexofenadine hydrochloride in pharmaceutical formulations
Kozan I, et al
Turkish Journal of Pharmaceutical Sciences, 5(3), 175-189 (2008)
Fexofenadine Hydrochloride
USP42/NF37: United States Pharmacopeia and National Formulary
United States Pharmacopeia/National Formulary, 34(3)(1), 1828-1828 (2019)
RP-HPLC method for the quantitative determination of fexofenadine hydrochloride in coated tablets and human serum
Arayne MS, et al.
Medicinal Chemistry Research, 20(1), 55-61 (2011)
Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria
Harold SN, et al.
Annals of Allergy, Asthma & Immunology, 84(5), 517-522 (2000)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務