C-152
Cannabidivarinic Acid (CBDVA) solution
1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
cannabis testing
cannabis testing
format
single component solution
storage temp.
−70°C
SMILES string
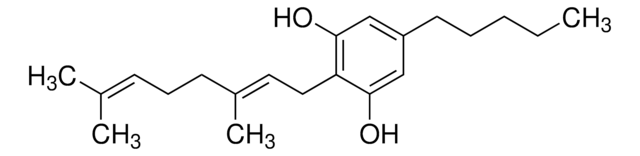
CCCC1=CC(O)=C([C@@H]2C=C(C)CC[C@H]2C(C)=C)C(O)=C1C(O)=O
InChI
1S/C20H26O4/c1-5-6-13-10-16(21)18(19(22)17(13)20(23)24)15-9-12(4)7-8-14(15)11(2)3/h9-10,14-15,21-22H,2,5-8H2,1,3-4H3,(H,23,24)/t14?,15-/m1/s1
InChI key
CZXWOKHVLNYAHI-YSSOQSIOSA-N
General description
Cannabidivarinic acid (CBDVA) is a non-psychoactive cannabinoid found in Cannabis that reportedly has anti-inflammatory properties.
Application
- Serum analysis to identify and quantify 18 phytocannabinoids by liquid chromatography-tandem mass spectrometry (LC-MS/MS)
- Quantitative analysis of 17 cannabinoids in cannabis and hemp samples by LC-MS/MS following their liquid-solid extraction following AOAC and ASTM guidelines for cannabis and hemp matrices
- Liquid chromatographic (LC)-diode array detection (DAD) based separation and measurement of 20 cannabinoids from a variety of cannabis samples
- Untargeted analysis of extracts from cultivars of 23 Cannabis sativa L plant materials by gas chromatography–time-of-flight/mass spectrometry (GC-TOF/MS) and liquid chromatography quadrupole time-of-flight tandem mass spectrometry (LC-QTOF MS/MS)
Features and Benefits
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Legal Information
Related product
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
35.6 °F - closed cup
flash_point_c
2.0 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
We offer a portfolio of cannabinoid CRMs such as THC and THCA, available as single component solutions or mixes, certified in accordance with ISO 17034 and ISO/IEC 17025 for accurate cannabinoid profiling and potency testing.
A comprehensive high performance liquid chromatography-diode array detection (HPLC-DAD) workflow for the analysis of 14 cannabinoids in hemp bud extracts within 10 minutes using robust Chromolith® monolithic silica HPLC columns with low column back pressure.
A complete workflow for the HPLC separation of 17 cannabinoids in different cannabis products using Ascentis® Express C18 Column in a shorter run time with retention time stability. Learn more here.
Protocols
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromenic Acid (CBCA) solution, 1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL
HPLC separation of 17 important cannabinoids including CBD, delta 9 THC and THCA. Read the application note
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service