Key Documents
N9150
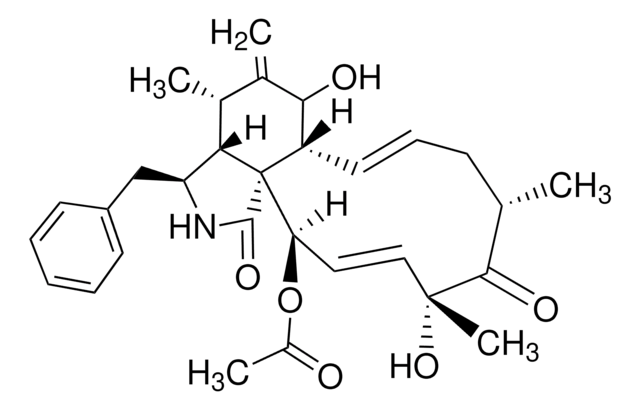
Nystatin
ready made solution, suitable for cell culture
Synonim(y):
Nystatin, Fungicidin, Mycostatin
About This Item
Polecane produkty
product name
Nystatin Ready made solution, suitable for cell culture
pochodzenie biologiczne
Streptomyces noursei
Postać
solution
metody
cell culture | mammalian: suitable
Warunki transportu
dry ice
temp. przechowywania
−20°C
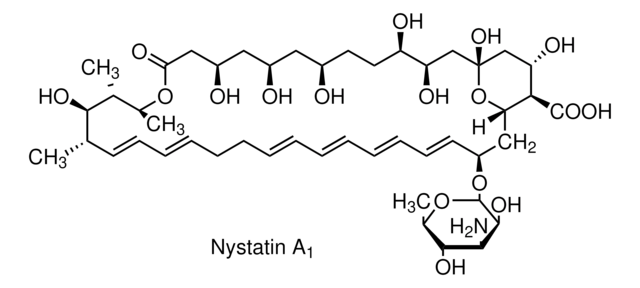
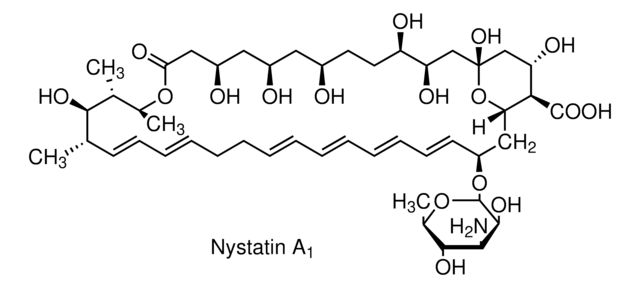
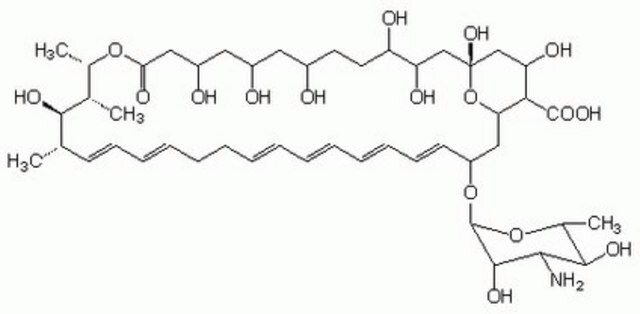
ciąg SMILES
O[C@@H]([C@H](C)[C@H](C)O1)[C@@H](C)/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@]3([H])[C@H](C(O)=O)[C@@H](O)C[C@](O)(O3)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)C[C@@H](O)CC1=O
InChI
1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28+,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1
Klucz InChI
VQOXZBDYSJBXMA-QEKUPDCNSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Działania biochem./fizjol.
Antimicrobial spectrum: Nystatin acts against fungi, yeasts and molds.
Cechy i korzyści
- Nystatin ready made solution is a fully solubilized formulation of Nystatin with potency of 8000-13000 units/ml according to USP standard.
- Nystatin ready made solution is 0.2μm filtered, endotoxin.
- The suggested working concentration of Nystatin ready made solution is 30 units/ml.
- The ready made solution is light sensitive. It is recommended to store the solution in the dark.
- It is recommended to avoid freeze thaw cycles of this product. Therefore, it is recommended to aliquot and store at -20°C.
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej