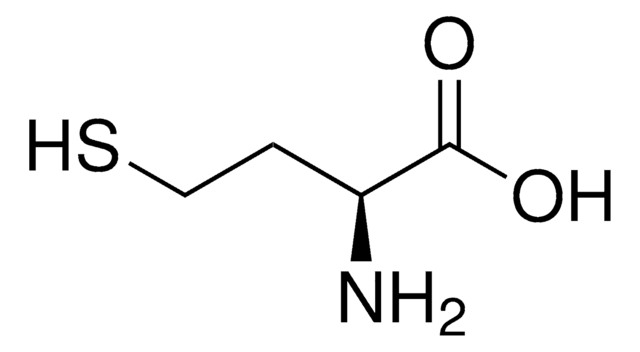
L8543
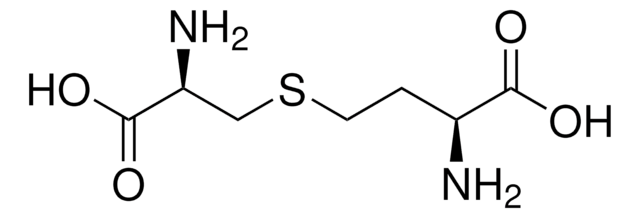
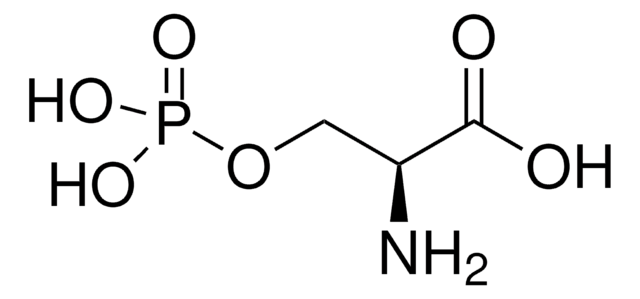
DL-Lanthionine
≥98% (TLC)
Synonim(y):
DL-3,3′-Thiodialanine, S-(2-Amino-2-carboxyethyl)-DL-cysteine
About This Item
Polecane produkty
Nazwa produktu
DL-Lanthionine, ≥98% (TLC)
Poziom jakości
Próba
≥98% (TLC)
Formularz
powder
kolor
white
rozpuszczalność
1 M HCl: soluble
Zastosowanie
detection
ciąg SMILES
S(CC(N)C(=O)O)CC(N)C(=O)O
InChI
1S/C6H12N2O4S/c7-3(5(9)10)1-13-2-4(8)6(11)12/h3-4H,1-2,7-8H2,(H,9,10)(H,11,12)
Klucz InChI
DWPCPZJAHOETAG-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
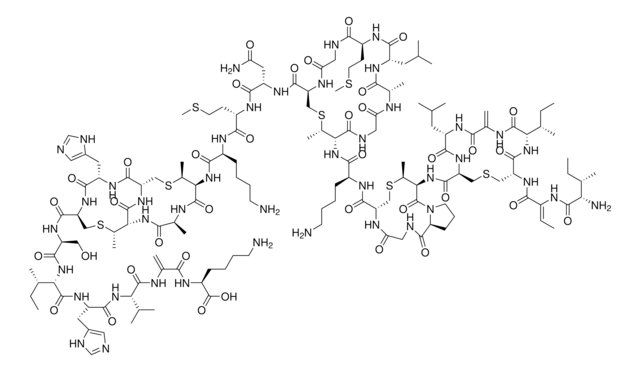
- Synteza lantybiotyku laktocyny S z wykorzystaniem cyklizacji peptydów w fazie stałej...: Badania te podkreślają innowacyjne metody syntezy antybiotyków opartych na peptydach, takich jak laktocyna S, wykorzystujące dl-lanthioninę do tworzenia kluczowych wiązań tioeterowych, które zwiększają aktywność i stabilność tych peptydów (Ross et al., 2010).
- Gamma-laza cystationinowa Streptomyces phaeochromogenes...: Badania te dokumentują izolację i charakterystykę enzymu przetwarzającego cystationinę, który obejmuje dl-lanthioninę jako analog strukturalny, ujawniając jej rolę w fizjologii bakterii i potencjalne zastosowania w biotechnologii (Nagasawa et al., 1984).
- Dostępność dl-lanthioniny w celu promowania wzrostu u młodych szczurów po dodaniu do diety ubogiej w cystynę i metioninę...: To historyczne badanie bada wartość odżywczą dl-lanthioniny, badając jej zdolność do zastępowania niezbędnych aminokwasów w dietach wzrostowych, co pomaga w zrozumieniu jej potencjalnego zastosowania w suplementach diety (Jones et al., 1948).
Działania biochem./fizjol.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej