Key Documents
A9384
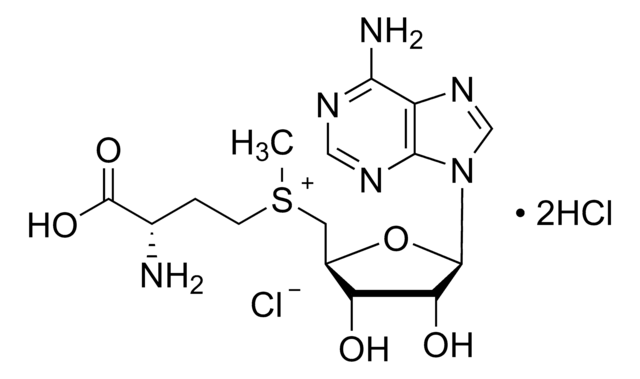
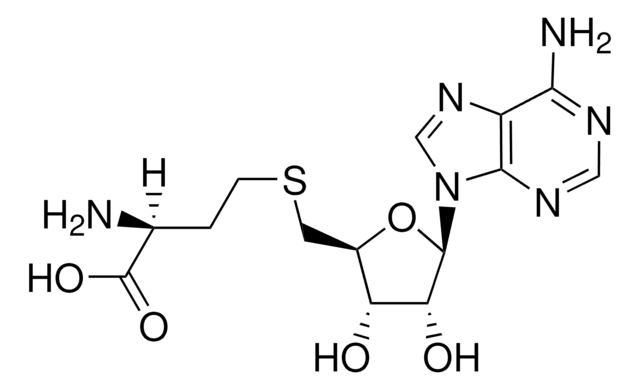
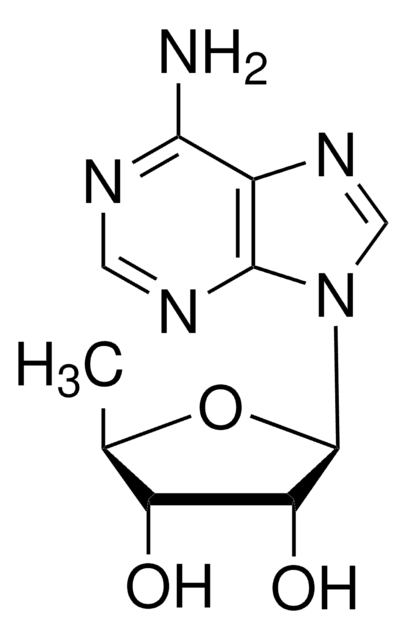
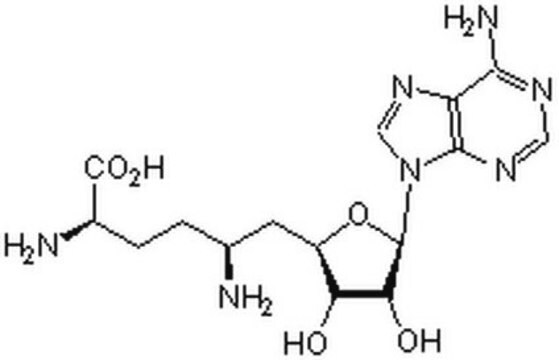
S-(5′-Adenosyl)-L-homocysteine
crystalline
Synonim(y):
5′-Deoxy-S-adenosyl-L-homocysteine, AdoHcy, S-(5′-Deoxyadenosine-5′)-L-homocysteine
About This Item
Polecane produkty
Próba
≥98.0% (HPLC)
≥98.0% (TLC)
Poziom jakości
Postać
crystalline
masa cząsteczkowa
384.41
rozpuszczalność
1 M HCl: soluble 19.60-20.40 mg/mL, clear to slightly hazy, colorless to faintly yellow
temp. przechowywania
−20°C
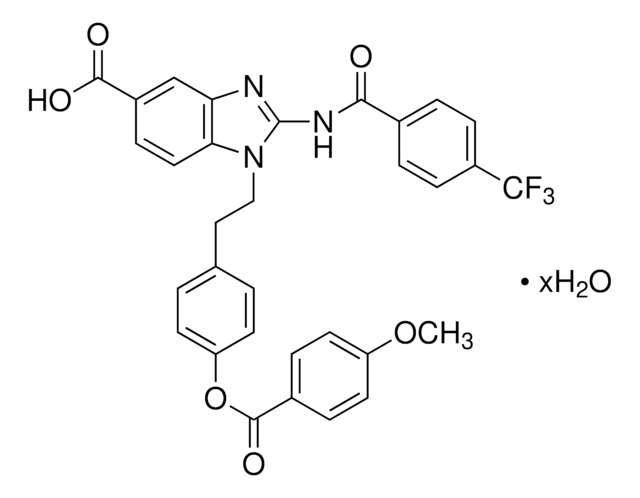
ciąg SMILES
N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23)C(O)=O
InChI
1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1
Klucz InChI
ZJUKTBDSGOFHSH-WFMPWKQPSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
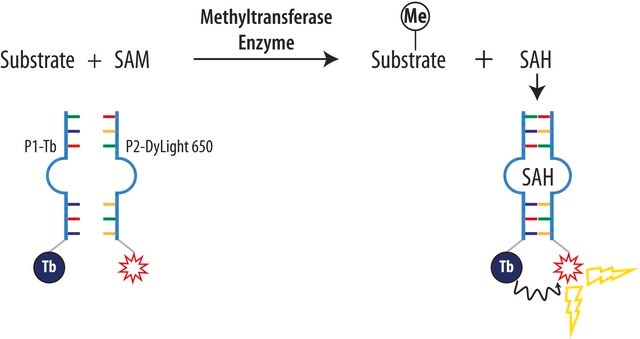
- to investigate whether AdoHcy competes with AdoMet in the down-regulation of reporter activity of LUC reporter gene
- as a reagent to study the abundance patterns of SAH and its correlation with vertebrate metamorphosis
- in the optimization of the protein (lysine K) methyltransferase SET7/9 activity assay
- in the fluorescence polarization (FP) assay during dengue virus methyltransferase activity measurement
- as a standard for the measurement of SAH from blood samples by high performance liquid chromatography (HPLC) with fluorimetric detection method
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej