F9813
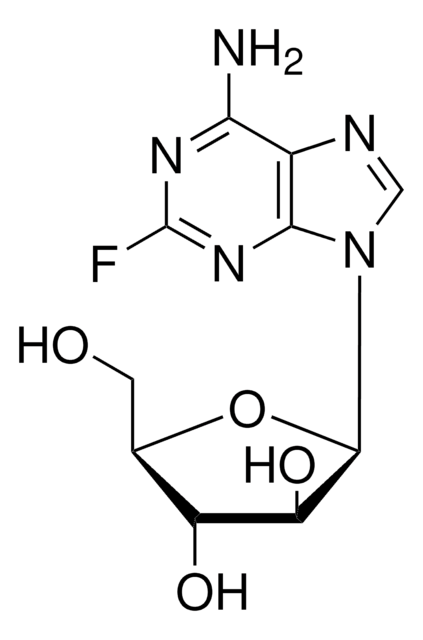
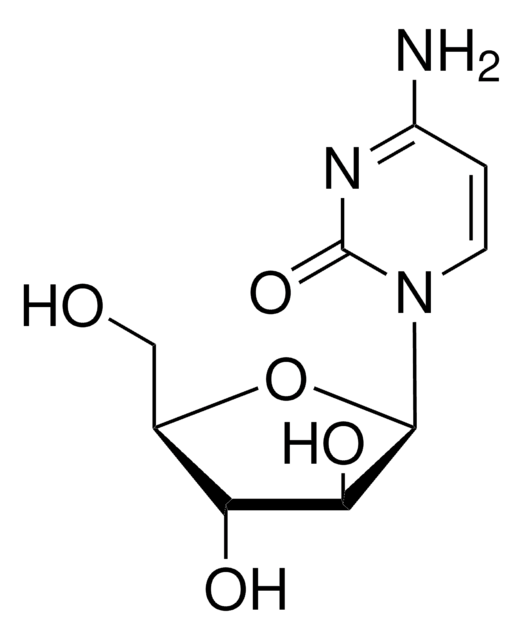
Fludarabine phosphate
Synonim(y):
2-Fluoro-9-(5-O-phosphono-β-D-arabinofuranosyl)-9H-purin-6-amine
About This Item
Polecane produkty
Formularz
powder
Poziom jakości
kolor
white
rozpuszczalność
DMSO: soluble
spektrum działania antybiotyku
neoplastics
Tryb działania
DNA synthesis | interferes
temp. przechowywania
−20°C
InChI
1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)
Klucz InChI
GIUYCYHIANZCFB-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
- Characterization of Chemical Interactions between Clinical Drugs and the Oral Bacterium, Corynebacterium matruchotii, via Bioactivity-HiTES.: This study explores the interactions of clinical drugs like Fludarabine phosphate with Corynebacterium matruchotii, highlighting potential impacts on oral microbiota and implications for drug efficacy and safety (Lee DY et al., 2024).
- Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours.: Investigates the efficacy of a combination of Fludarabine phosphate with other chemotherapeutics delivered via nanocarriers, aiming to enhance treatment outcomes for pancreatic cancer by improving drug delivery to the tumor site (Rudolph D et al., 2024).
- Macrophage neogenin deficiency exacerbates myocardial remodeling and inflammation after acute myocardial infarction through JAK1-STAT1 signaling.: This research demonstrates the role of Fludarabine phosphate in modulating inflammation and cardiac repair post-myocardial infarction, offering insights into its potential therapeutic benefits beyond oncology (Zhang J et al., 2023).
- SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis.: Discusses the cellular mechanisms by which Fludarabine phosphate may influence metabolic pathways in cancer cells, highlighting its potential to disrupt tumor metabolism and promote cancer cell death (Li Y et al., 2023).
- CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome.: Reviews the use of Fludarabine phosphate in preconditioning regimens for CAR T-cell therapy, emphasizing its role in enhancing the efficacy of immunotherapy in treating autoimmune disorders (Pecher AC et al., 2023).
Działania biochem./fizjol.
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Muta. 2 - Repr. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej