Kluczowe dokumenty
F3627
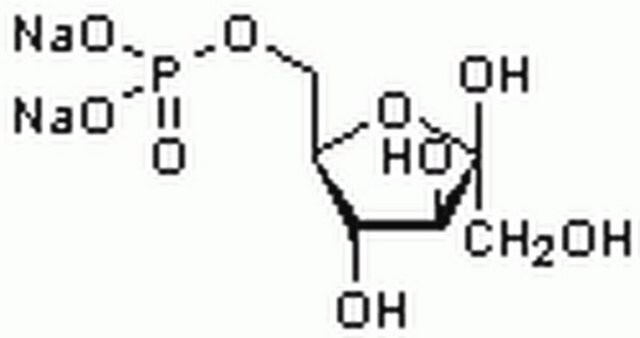
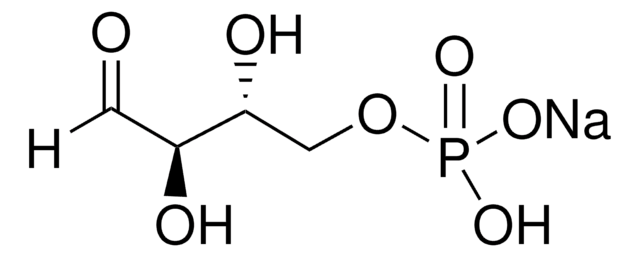
D-Fructose 6-phosphate disodium salt hydrate
≥98%, amorphous powder
Synonim(y):
Fosforan (2R,3R,4S)-2,3,4,6-tetrahydroksy-5-oksoheksylu sodu
About This Item
Polecane produkty
pochodzenie biologiczne
bacterial (Corynebacterium)
Poziom jakości
Próba
≥98%
Formularz
amorphous powder
zanieczyszczenia
<0.05 mol % fructose 1,6-diphosphate
<1.5 mol % glucose 6-phosphate
kolor
white to off-white
rozpuszczalność
H2O: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
ślady kationów
Na: 14.6-15.6% (dry basis)
Zastosowanie
agriculture
temp. przechowywania
−20°C
ciąg SMILES
O.[Na+].[Na+].OC[C@@]1(O)O[C@H](COP([O-])([O-])=O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13O9P.2Na.H2O/c7-2-6(10)5(9)4(8)3(15-6)1-14-16(11,12)13;;;/h3-5,7-10H,1-2H2,(H2,11,12,13);;;1H2/q;2*+1;/p-2/t3-,4-,5+,6-;;;/m1.../s1
Klucz InChI
VSCMQICEHMPOEC-HTKRKRNRSA-L
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Działania biochem./fizjol.
Inne uwagi
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Review the 10 steps of glycolysis in the Embden-Meyerhof-Parnas glycolytic pathway. Easily compare reaction stages and buy the enzymes for your life science research.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej