Wszystkie zdjęcia(1)
Kluczowe dokumenty
C6154
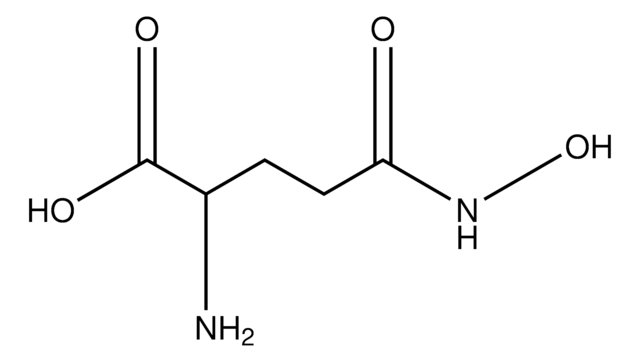
Z-Gln-Gly
γ-glutamyl donor substrate
Synonim(y):
N2-[(phenylmethoxy)carbonyl]-L-glutaminyl-glycine
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C15H19N3O6
Numer CAS:
Masa cząsteczkowa:
337.33
Numer MDL:
Kod UNSPSC:
12352209
Identyfikator substancji w PubChem:
NACRES:
NA.26
Polecane produkty
Poziom jakości
Formularz
powder
temp. przechowywania
−20°C
ciąg SMILES
NC(=O)CCC(NC(=O)OCc1ccccc1)C(=O)NCC(O)=O
InChI
1S/C15H19N3O6/c16-12(19)7-6-11(14(22)17-8-13(20)21)18-15(23)24-9-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,16,19)(H,17,22)(H,18,23)(H,20,21)
Klucz InChI
SOUXAAOTONMPRY-UHFFFAOYSA-N
Amino Acid Sequence
Z-Gln-Gly
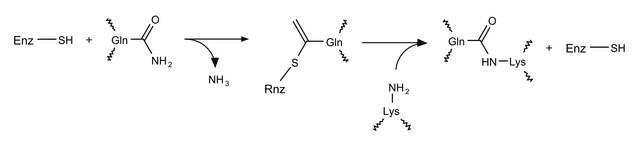
Zastosowanie
γ-Glutamyl donor substrate used in spectrophotometric determination of transglutaminase (TGase) activity. Z-Gln-Gly was used to enzymatically synthesize N-linked neoglycoproteins.
Działania biochem./fizjol.
N-Benzyloxycarbonyl-L-Glutaminylglycine (Z-Gln-Gly, Z-QG) is used as a substrate to differentiate and characterize transglutaminase(s) (TGase) that catalyzes the post-translational covalent cross-linking of Gln- and Lys-containing peptides. Z-QG supports glutamyl-level cross-linking applications thruough surface modification.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
D Ramos et al.
The Journal of organic chemistry, 66(9), 2948-2956 (2001-04-28)
A novel methodology for the enzymatic preparation from suitably derivatized oligosaccharides of N-linked neoglycopeptides using the microbial glutaminyl-peptide gamma-glutamyl transferase, transglutaminase (TGase), is described. N-Allyl glycosides of various oligosaccharides were photochemically coupled with cysteamine to yield amino-terminated thioether spacers, which
Kyunga Sung et al.
Biotechnology journal, 5(5), 456-462 (2010-03-12)
A chemically modified glass surface displaying a glutamyl donor substrate peptide (Z-QG) was developed for microbial transglutaminase (MTG)-mediated immobilization of recombinant proteins tagged with an MTG-reactive lysine-containing substrate peptide (K-tag). To evaluate the surface modification conditions affecting the enzymatic protein
Dongdong Mu et al.
Applied microbiology and biotechnology, 102(13), 5533-5543 (2018-04-25)
Microbial transglutaminase (MTG) from Streptomyces mobaraensis has been widely used for crosslinking proteins in order to acquire products with improved properties. To improve the yield and enable a facile and efficient purification process, recombinant vectors, harboring various heterologous signal peptide-encoding
Rui P Queirós et al.
Food research international (Ottawa, Ont.), 115, 73-82 (2019-01-03)
Microbial transglutaminase (MTG) is an enzyme largely used in the food industry, mainly to improve food texture. However, many globular proteins show low susceptibility to the action of this enzyme. High-pressure processing (HPP), being able to change protein conformation, may
Ahmed Besheer et al.
Journal of pharmaceutical sciences, 98(11), 4420-4428 (2009-01-22)
Polymer-drug and polymer-protein conjugates are promising candidates for the delivery of therapeutic agents. PEGylation, using poly(ethylene glycol) for the conjugation, is now the gold standard in this field, and some PEGylated proteins have successfully reached the market. Hydroxyethyl starch (HES)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej