Wszystkie zdjęcia(1)
Kluczowe dokumenty
75762
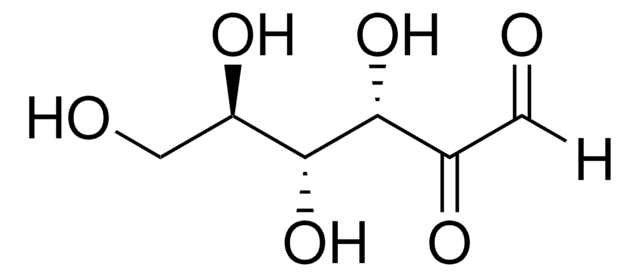
3-Deoxyglucosone
≥75% (TLC)
Synonim(y):
3-Deoxy-D-erythro-hexosulose
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C6H10O5
Numer CAS:
Masa cząsteczkowa:
162.14
Numer MDL:
Kod UNSPSC:
12352201
Identyfikator substancji w PubChem:
NACRES:
NA.25
Polecane produkty
Poziom jakości
Próba
≥75% (TLC)
Formularz
solid
kolor
faintly yellow to orange
temp. przechowywania
room temp
ciąg SMILES
OC[C@H]1OC(O)C(=O)C[C@@H]1O
InChI
1S/C6H10O5/c7-2-5-3(8)1-4(9)6(10)11-5/h3,5-8,10H,1-2H2/t3-,5+,6?/m0/s1
Klucz InChI
UHPMJDGOAZMIID-OPAZFOKUSA-N
Zastosowanie
3-Deoxyglucosone is used as a reference for the analysis and detection of glucose degradation products, glycating agents, generated by processes such as heat sterilization.
Inne uwagi
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Michael Hellwig et al.
Journal of agricultural and food chemistry, 58(19), 10752-10760 (2010-09-09)
1,2-Dicarbonyl compounds are formed in food during Maillard and caramelization reactions. 3-Deoxy-D-threo-hexos-2-ulose (3-deoxygalactosone, 3-DGal) and galactosone, two 1,2-dicarbonyl compounds originating from the degradation of galactose, were synthesized and converted to the respective quinoxalines, which were characterized by NMR spectroscopy. Analytical
He Li et al.
Journal of agricultural and food chemistry, 67(32), 9050-9059 (2019-07-25)
The control of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP) formation in the Maillard reaction is important to improve the thermally treated food quality as a result of its intense bitterness and potential toxicity. In this work, phenolic acids, such as gallic, protocatechuic, caffeic, and
Danielle T Loughlin et al.
Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 17(5), 739-749 (2009-09-23)
The interaction of fibroblasts with the extracellular matrix is critical for wound healing. Advanced glycation end products (AGEs) occur through nonenzymatic glycation of long-lived proteins such as collagens. One precursor to these modifications, 3-deoxyglucosone (3DG), is elevated in patients with
Alina Shapira et al.
Molecular nutrition & food research, 51(4), 473-478 (2007-03-29)
Peritoneal dialysis (PD) is commonly performed by using preprepared dialysis solutions containing glucose, which are thermally treated to achieve commercial sterilization. A series of glucose degradation products (GDPs) are being formed, which react with the tissue during the dialysis procedure
Martin Erixon et al.
Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis, 28(3), 277-282 (2008-05-14)
Glucose degradation products (GDPs) are important in the outcome of peritoneal dialysis (PD) treatment. 3,4-dideoxyglucosone-3-ene (3,4-DGE) is the most cytotoxic GDP found in conventionally manufactured fluids and may, in addition, be recruited from 3-deoxyglucosone (3-DG). It is not known what
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej