Kluczowe dokumenty
PHR1114
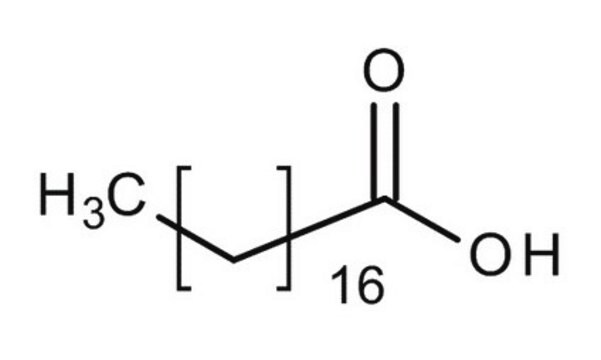
Stearic Acid
Pharmaceutical Secondary Standard; Certified Reference Material
Synonim(y):
Stearic acid, 1-Heptadecanecarboxylic acid, C18:0, Cetylacetic acid, NSC 25956, NSC 261168, Octadecanoic acid, Stearophanic acid
About This Item
Polecane produkty
klasa czystości
certified reference material
pharmaceutical secondary standard
Poziom jakości
agency
traceable to Ph. Eur. S1340000
traceable to USP 1621008
ciśnienie pary
1 mmHg ( 173.7 °C)
rodzina API
stearic acid
Certyfikat analizy
current certificate can be downloaded
metody
HPLC: suitable
gas chromatography (GC): suitable
bp
361 °C (lit.)
mp
67-72 °C (lit.)
Zastosowanie
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical (small molecule)
Format
neat
temp. przechowywania
2-30°C
ciąg SMILES
CCCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C18H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h2-17H2,1H3,(H,19,20)
Klucz InChI
QIQXTHQIDYTFRH-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Zastosowanie
Komentarz do analizy
Inne uwagi
Przypis
Polecane produkty
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
nwg
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Protokoły
HPLC Analysis of Free Fatty Acids on SUPELCOSIL™ LC-8
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej