426288
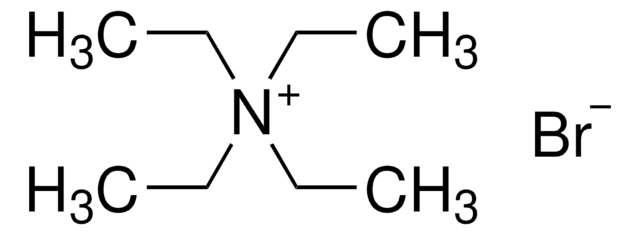
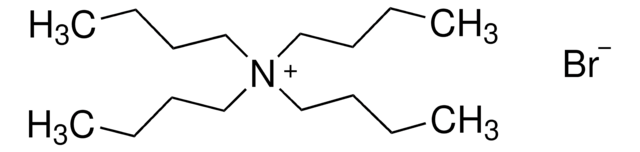
Tetrabutylammonium bromide
ACS reagent, ≥98.0%
Synonim(y):
N,N,N-tributyl-1-butanaminium bromide, TBAB, TBABr, tetra-n-butylammonium bromide
About This Item
Polecane produkty
klasa czystości
ACS reagent
Poziom jakości
Próba
≥98.0%
Postać
solid
charakterystyka ekologicznej alternatywy
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
zanieczyszczenia
≤0.5% tributylamine hydrobromide
≤0.5% tributylamine
mp
102-106 °C (lit.)
ciąg SMILES
[Br-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.BrH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
Klucz InChI
JRMUNVKIHCOMHV-UHFFFAOYSA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- Synthesis of (2S)-5-(3-phenyl-2-phthalimidylpropanoylamino)isophthalic acid.
- Synthesis of alkyl-substituted pyrroles in the absence of catalyst and organic solvent.
- Synthesis of dithioacetals from acetals by transthioacetalisation in a solvent free environment.
- Synthesis of polyamides (PAs) by the polymerization of terephthalic acid and diisocyanates.
- Catalyze the addition of thiols to conjugated alkenes.
- Dehydrochlorination of poly(vinyl chloride).
Process for Producing Halogenated Heteroaryl Compounds
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej
![4-[4-(Dimethylamino)styryl]pyridine 95%](/deepweb/assets/sigmaaldrich/product/structures/225/605/ad18cc93-9d43-467b-8618-105948f9692b/640/ad18cc93-9d43-467b-8618-105948f9692b.png)

![trans-4-[4-(Dimethylamino)styryl]-1-methylpyridinium iodide Dye content 98 %](/deepweb/assets/sigmaaldrich/product/structures/416/722/5d59b6c3-5f2d-4396-a721-5cb82ba7038c/640/5d59b6c3-5f2d-4396-a721-5cb82ba7038c.png)