Key Documents
TRYPSEQM-RO
Roche
Trypsin Sequencing Grade, modified
from bovine pancreas
Synonim(y):
Trypsin
About This Item
Polecane produkty
pochodzenie biologiczne
bovine pancreas
Poziom jakości
klasa czystości
protein sequencing grade
Postać
lyophilized (salt-free)
masa cząsteczkowa
24.000 g/mol
opakowanie
pkg of 4 × 100 μg (11418033001)
pkg of 4 × 25 μg (11418025001)
producent / nazwa handlowa
Roche
warunki przechowywania
(Keep container tightly closed in a dry and well-ventilated place.)
stężenie
0.01-0.2 % (w/w)
metody
protein sequencing: suitable
zanieczyszczenia
Chymotrypsin
kolor
white
optymalne pH
8.0
rozpuszczalność
10 g/L
przydatność
suitable for protein modification
numer dostępu UniProt
Zastosowanie
life science and biopharma
obecność zanieczyszczeń
Contaminating activities corresponds
Chymotrypsin , contains
temp. przechowywania
2-8°C
informacje o genach
cow ... PRSS1(780933)
Powiązane kategorie
Opis ogólny
Trypsin is a highly efficient and specific protease widely used in proteomics for protein digestion. It produces short peptides with specific characteristics that are compatible with current separation and identification methods such as liquid chromatography, mass spectrometry (MS).
Inhibitors: TLCK, DFP, PMSF, leupeptin, soybean trypsin inhibitor, trypsin inhibitor from hen egg, aprotinin, α2-macroglobulin,α1-antitrypsin, APMSF, and antipain.
Specyficzność
Zastosowanie
It is used for:
- Protein-structure elucidation
- Tryptic mapping
- Fingerprinting analysis
- Sequence analysis
- Translocation studies
- Protein identification
- Protein digestion during lipoprotein preparation for liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Jakość
Uwaga dotycząca przygotowania
Storage conditions (working solution): -15 to -25 °C
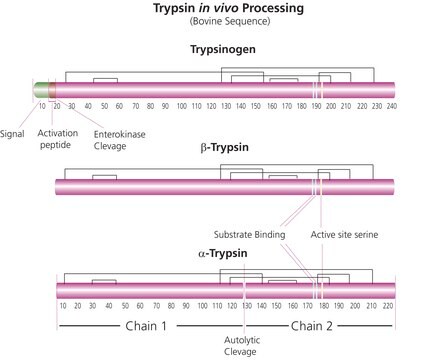
Trypsin Sequencing Grade, modified, is more resistant to autolysis, even at pH values in the neutral and weakly basic range. The enzyme can be used in high concentrations.
A solution in 1% acetic acid or 1 mM HCI can be used for up to one week when stored at 2 to 8° C. Stored in aliquots at -15 to -25 °C, the solution is stable for at least one year without loss of activity.
Przechowywanie i stabilność
Inne uwagi
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej