344282
Forskolin
from Coleus forskohlii, ≥97% (HPLC), liquid, positive inotropic agent, Calbiochem
Synonim(y):
Forskolin, Coleus forskohlii in DMSO
About This Item
Polecane produkty
Nazwa produktu
Forskolin, Coleus forskohlii in DMSO, The major cell-permeable diterpene isolated from the Indian plant Coleus forskohlii.
Poziom jakości
Próba
≥97% (HPLC)
Formularz
liquid
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
desiccated (hygroscopic)
protect from light
Warunki transportu
wet ice
temp. przechowywania
−20°C
Opis ogólny
Opakowanie
Ostrzeżenie
Postać fizyczna
Rekonstytucja
Inne uwagi
Noveen, A., et al. 1996. Biochem. Biophys. Res. Commun.219, 180.
Galli, C., et al. 1995. J. Neurosci.15, 1172.
Li, X., et al. 1995. Am. J. Physiol.269, C986.
Lomo, J., et al. 1995. J. Immunol.154, 1634.
Uneyama, H., et al. 1993. J. Biol. Chem.268, 168.
Laurenza, A., et al. 1989. Trends Pharmacol. Sci.10, 442.
Adashi, E.Y., and Resnick, C.E. 1986. J. Cell. Biochem.31, 217.
Seamon, K.B., and Daly, J.W. 1986. Adv. Cyclic Nucleotide Protein Phosphorylation Res.20, 1.
Huang, R., et al. 1982. Cyclic Nucleotide Res.8, 385.
Metzger, H., and Lindner, E. 1981. IRCS Med. Sci. Biochem. Cardiovasc. System Pharmacol.9, 99.
Informacje prawne
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
188.6 °F - (refers to pure substance)
Temperatura zapłonu (°C)
87 °C - (refers to pure substance)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
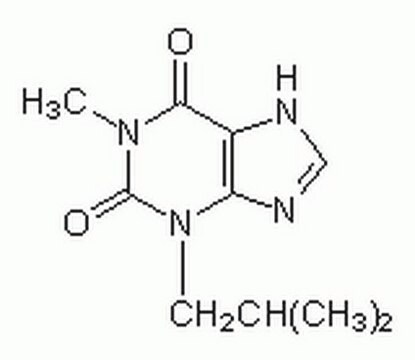
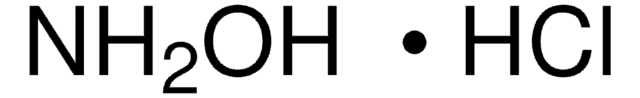
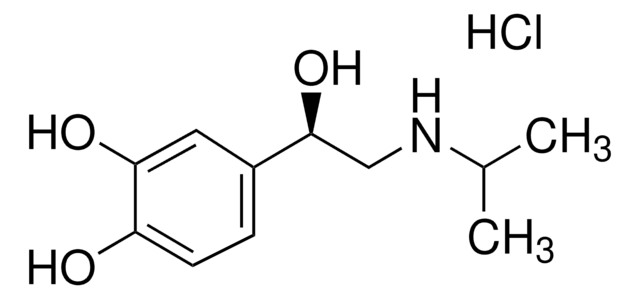
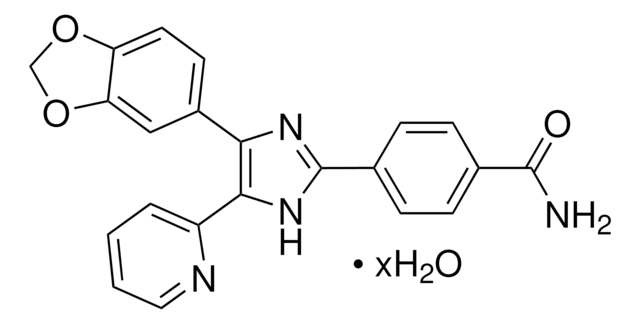
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej