Wszystkie zdjęcia(1)
Key Documents
860657P
Avanti
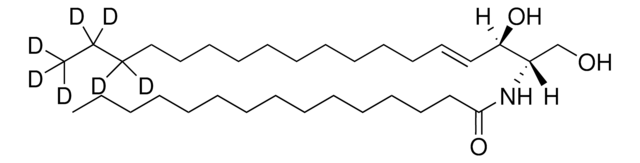
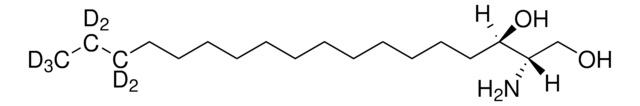
sphingosine-d7
Avanti Research™ - A Croda Brand 860657P, powder
Synonim(y):
D-erythro-sphingosine-d7
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C18H30D7NO2
Numer CAS:
Masa cząsteczkowa:
306.54
Kod UNSPSC:
12352211
NACRES:
NA.25
Polecane produkty
Postać
powder
opakowanie
pkg of 1 × 1 mg (860657P-1mg)
pkg of 1 × 5 mg (860657P-5mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand 860657P
Warunki transportu
dry ice
temp. przechowywania
−20°C
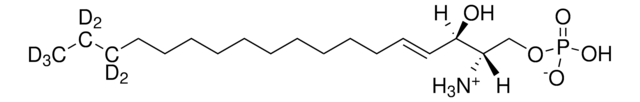
ciąg SMILES
OC[C@@](N)([H])[C@@](O)([H])/C=C/CCCCCCCCCCC(C(C([2H])([2H])[2H])([2H])[2H])([2H])[2H]
Opis ogólny
Sphingosine-d7 is a deuterated derivative of sphingosine. Sphingosine is an important bioactive sphingolipid metabolite. It is a 18-carbon amino alcohol derived from sphingomyelin. Sphingosine contains an unsaturated hydrocarbon chain.
Zastosowanie
Sphingosine-d7 has been used as an internal standard in liquid chromatography–tandem mass spectrometry for quantitative analysis of sphingolipids in biological samples.
Działania biochem./fizjol.
Sphingosine negatively regulates cell proliferation and induces apoptosis. It has an ability to regulate the activities of phospholipases, protein kinases, ion channels, cannabinoid receptor type 1 (CB-1) receptors and steroidogenic factor 1 (SF-1) receptors. Sphingosine acts as a precursor for ceramide synthesis.
Opakowanie
5 mL Amber Glass Screw Cap Vial (860657P-1mg)
5 mL Amber Glass Screw Cap Vial (860657P-5mg)
Informacje prawne
Avanti Research is a trademark of Avanti Polar Lipids, LLC
This page may contain text that has been machine translated.
najczęściej kupowane z tym produktem
Numer produktu
Opis
Cennik
Kod klasy składowania
11 - Combustible Solids
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Henning Carstens et al.
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 53(6), 1015-1028 (2019-12-20)
Pulmonary infections with Pseudomonas aeruginosa (P. aeruginosa) or Staphylococcus aureus (S. aureus) are of utmost clinical relevance in patients with cystic fibrosis, chronic obstructive pulmonary disease, after trauma and burn, upon ventilation or in immuno-compromised patients. Many P. aeruginosa and
Stephanie Schwalm et al.
The American journal of pathology, 187(11), 2413-2429 (2017-08-16)
Kidney fibrosis is a hallmark of chronic kidney disease and leads to extracellular matrix accumulation, organ scarring, and loss of kidney function. In this study, we investigated the role of sphingosine kinase-2 (SPHK2) on the progression of tubular fibrosis by
Iulia Zoicas et al.
Cells, 9(5) (2020-05-24)
Human and murine studies identified the lysosomal enzyme acid sphingomyelinase (ASM) as a target for antidepressant therapy and revealed its role in the pathophysiology of major depression. In this study, we generated a mouse model with overexpression of Asm (Asm-tgfb)
Irina Alecu et al.
Journal of lipid research, 58(1), 60-71 (2016-11-23)
The 1-deoxysphingolipids (1-deoxySLs) are atypical sphingolipids (SLs) that are formed when serine palmitoyltransferase condenses palmitoyl-CoA with alanine instead of serine during SL synthesis. The 1-deoxySLs are toxic to neurons and pancreatic β-cells. Pathologically elevated 1-deoxySLs cause the inherited neuropathy, hereditary
Olivier Cuvillier
Biochimica et biophysica acta, 1585(2-3), 153-162 (2003-01-18)
The sphingolipid metabolites ceramide, sphingosine, and sphingosine 1-phosphate contribute to controlling cell proliferation and apoptosis. Ceramide and its catabolite sphingosine act as negative regulators of cell proliferation and promote apoptosis. Conversely, sphingosine 1-phosphate, formed by phosphorylation of sphingosine by a
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej