800814P
Avanti
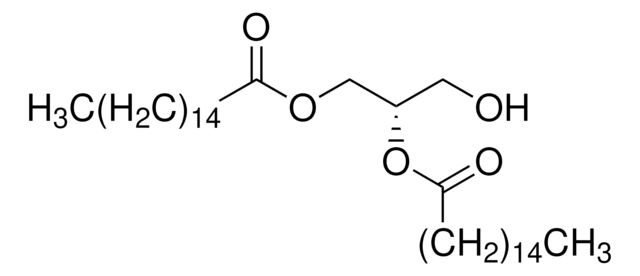
14:0 DG
1,2-dimyristoyl-sn-glycerol, powder
Synonim(y):
1,2-ditetradecanoyl-sn-glycerol; DG(14:0/14:0/0:0)
About This Item
Polecane produkty
Próba
>99% (TLC)
Formularz
powder
opakowanie
pkg of 1 × 10 mg (800814P-10mg)
pkg of 1 × 25 mg (800814P-25mg)
producent / nazwa handlowa
Avanti Research™ - A Croda Brand 800814P
typ lipidu
neutral glycerides
neutral lipids
Warunki transportu
dry ice
temp. przechowywania
−20°C
ciąg SMILES
O([C@H](COC(=O)CCCCCCCCCCCCC)CO)C(=O)CCCCCCCCCCCCC
InChI
1S/C31H60O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-30(33)35-28-29(27-32)36-31(34)26-24-22-20-18-16-14-12-10-8-6-4-2/h29,32H,3-28H2,1-2H3/t29-/m0/s1
Klucz InChI
JFBCSFJKETUREV-LJAQVGFWSA-N
Opis ogólny
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Zastosowanie
- in the reconstitution of dry lipids for thin layer chromatography
- in lipid nanoparticles for RNA delivery studies
- as a standard in gas chromatography–mass spectrometry (GC-MS) analysis for the quantification of lipid A diacylglycerols
Działania biochem./fizjol.
Opakowanie
Przechowywanie i stabilność
Inne uwagi
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej
![23:2 Diyne PE [DC(8,9)PE] 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine, powder](/deepweb/assets/sigmaaldrich/product/images/228/422/4e95f75c-14fa-4117-a383-2eff73fa927f/640/4e95f75c-14fa-4117-a383-2eff73fa927f.jpg)