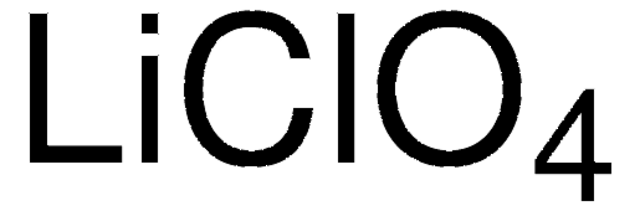931969
Lithium perchlorate
anhydrous, ≥99.9% trace metals basis
Synonim(y):
Perchloric acid lithium salt
About This Item
Polecane produkty
klasa czystości
anhydrous
battery grade
Poziom jakości
Próba
≥99.9% trace metals basis
Postać
powder
charakterystyka ekologicznej alternatywy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
zanieczyszczenia
≤1000 ppm (trace metals analysis)
pH
6.0-7.5 (25 °C, 5%, aq.sol.)
mp
236 °C (lit.)
rozpuszczalność
H2O: 59.8 g/dL at 25 °C
ślady anionów
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
ślady kationów
Fe: ≤5 ppm
heavy metals: ≤10 ppm
Zastosowanie
battery manufacturing
kategoria ekologicznej alternatywy
ciąg SMILES
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
Klucz InChI
MHCFAGZWMAWTNR-UHFFFAOYSA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Zastosowanie
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Opakowanie
500g in poly bottle
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
5.1A - Strongly oxidizing hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej