Kluczowe dokumenty
930423
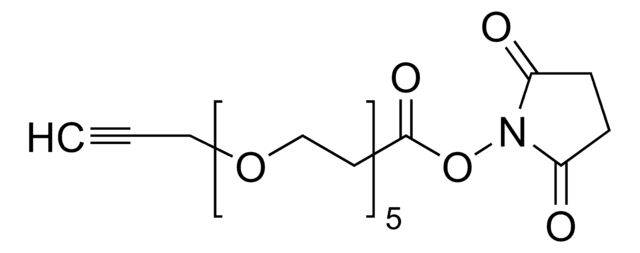
CP-alkyne
≥95%
Synonim(y):
tetrafluoroboran 2,4,6-trimetylo-1-(metylo((pent-4-yn-1-yloksy)karbonylo)amino)pirydyn-1-ium
About This Item
Polecane produkty
opis
Application: Chemoproteomics
Poziom jakości
Próba
≥95%
Formularz
liquid
temp. przechowywania
−20°C
ciąg SMILES
CC1=CC(C)=[N+](N(C(OCCCC#C)=O)C)C(C)=C1.F[B-](F)(F)F
Klucz InChI
JEYOQLIMTNLUGZ-UHFFFAOYSA-N
Zastosowanie
Inne uwagi
2. Ilościowa platforma profilowania reaktywności tiolowej do analizy proteomów cystein reaktywnych redoks i elektrofilowo
3. Etynylacja reszt cysteinowych: Od peptydów do białek in vitro i w żywych komórkach
4. Platforma chemoproteomiczna do oceny potencjału bioaktywacji leków
5. Inhibicja zależnych od cynku deacetylaz histonowych za pomocą chemicznie wyzwalanego elektrofilu
6. Odwracalność kowalencyjnych adduktów elektrofil-białko i toksyczność chemiczna
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej