Kluczowe dokumenty
595314
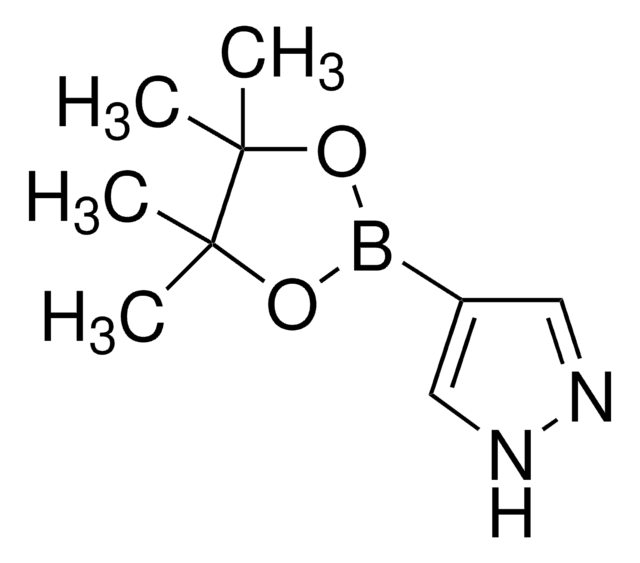
1-Methylpyrazole-4-boronic acid pinacol ester
95%
Synonim(y):
1-Methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole, 1-Methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, 1-Methyl-4-pyrazoleboronic acid pinacol ester, 2-(1-Methylpyrazol-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-methylpyrazole
About This Item
Polecane produkty
Poziom jakości
Próba
95%
Formularz
solid
mp
59-64 °C (lit.)
ciąg SMILES
Cn1cc(cn1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C10H17BN2O2/c1-9(2)10(3,4)15-11(14-9)8-6-12-13(5)7-8/h6-7H,1-5H3
Klucz InChI
UCNGGGYMLHAMJG-UHFFFAOYSA-N
Powiązane kategorie
Zastosowanie
- Suzuki-Miyaura cross-coupling reactions
- Transesterification reactions
Reagent used for preparation of
- Aminothiazoles as γ-secretase modulators
- Amino-pyrido-indol-carboxamides, as potential JAK2 inhibitors for myeloproliferative disorders therapy
- Pyridine derivatives as TGF-β1 and activin A signalling inhibitors
- MK-2461 analogs as inhibitors of c-Met kinase for the treatment of cancer
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej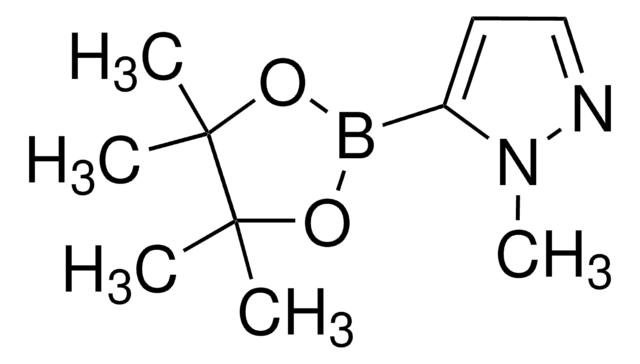
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![1-[1-(2-Methylphenyl)-1H-pyrazol-4-yl]methanamine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/193/071/8c363ad6-8306-4c4d-b322-749ff2feff6f/640/8c363ad6-8306-4c4d-b322-749ff2feff6f.png)
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrrolo[2,3-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/272/176/ea333f93-763d-458c-a328-3969b7d46e5d/640/ea333f93-763d-458c-a328-3969b7d46e5d.png)