Kluczowe dokumenty
440914
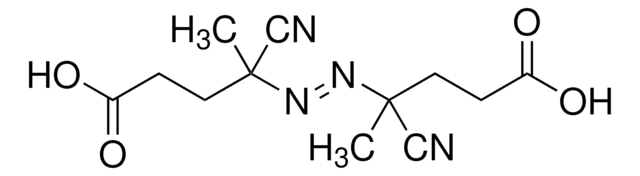
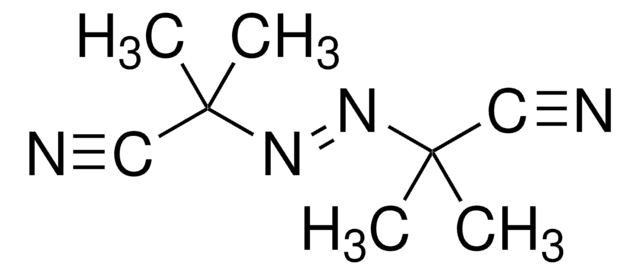
2,2′-Azobis(2-methylpropionamidine) dihydrochloride
powder or granules, 97%
Synonim(y):
α,α′-Azodiisobutyramidine dihydrochloride, AAPH
About This Item
Polecane produkty
Poziom jakości
Próba
97%
Formularz
powder or granules
t1/2
10 hr(56 °C)
mp
175-177 °C (lit.)
rozpuszczalność
acetone, dioxane, methanol, ethanol, DMSO and water: soluble
ciąg SMILES
Cl.Cl.CC(C)(\N=N\C(C)(C)C(N)=N)C(N)=N
InChI
1S/C8H18N6.2ClH/c1-7(2,5(9)10)13-14-8(3,4)6(11)12;;/h1-4H3,(H3,9,10)(H3,11,12);2*1H/b14-13+;;
Klucz InChI
LXEKPEMOWBOYRF-QDBORUFSSA-N
Powiązane kategorie
Zastosowanie
Polymerization initiator for acrylic, vinyl and allyl monomers.
Cechy i korzyści
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Self-heat. 1 - Skin Sens. 1
Kod klasy składowania
4.2 - Pyrophoric and self-heating hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Tools for Performing ATRP
We presents an article about Copper(I)-mediated Living Radical Polymerization in the Presence of Pyridylmethanimine Ligands, and the emergence of living radical polymerization mediated by transition metal catalysts in 1995, which was a seminal piece of work in the field of synthetic polymer chemistry.
Applying ARGET ATRP to the Growth of Polymer Brush Thin Films by Surface-initiated Polymerization
Protokoły
Sfery polimerowe służą jako szablony kryształów. Metody syntezy dają duże ilości.
Monodisperse, surfactant-free polymer spheres for use as colloidal crystal templates can be easily obtained in reasonably large quantities. Typical synthesis methods for poly(methyl methacrylate) (PMMA) and poly(styrene) (PS) by emulsifier free emulsion polymerization are described below and yield spheres several hundred nanometers in diameter.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 440914-100G | 4061835563098 |
| 440914-25G | 4061835515516 |
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej