Kluczowe dokumenty
417599
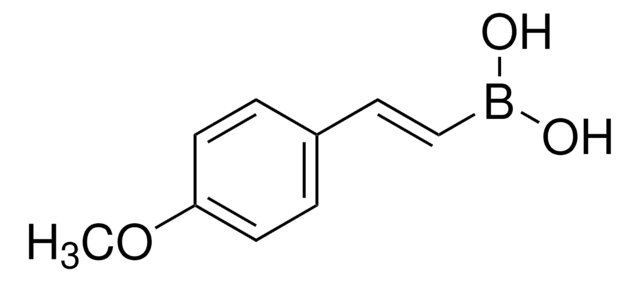
4-Methoxyphenylboronic acid
≥95.0%
Synonim(y):
(4-Methoxyphenyl)boric acid, (p-Methoxyphenyl)boronic acid, 4-Anisylboronic acid, 4-Methoxybenzeneboronic acid, p-Anisylboronic acid, p-Methoxybenzeneboronic acid
About This Item
Polecane produkty
Poziom jakości
Próba
≥95.0%
Postać
powder
mp
204-206 °C (lit.)
ciąg SMILES
COc1ccc(cc1)B(O)O
InChI
1S/C7H9BO3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5,9-10H,1H3
Klucz InChI
VOAAEKKFGLPLLU-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- Suzuki-Miyaura cross-coupling reactions
- Pd-catalyzed direct arylation
- Highly effective synthesis using palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water
- Palladium-catalyzed stereoselective Heck-type reaction
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Ruthenium catalyzed direct arylation
- Rh-catalyzed asymmetric conjugate addition
- Ligand-free copper-catalyzed coupling
Reagent used in Preparation of
- Palladium(II) thiocarboxamide complexes as Suzuki coupling catalyst
- Push-pull arylvinyldiazine chromophores with photophysical properties
Inne uwagi
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej