About This Item
Wzór empiryczny (zapis Hilla):
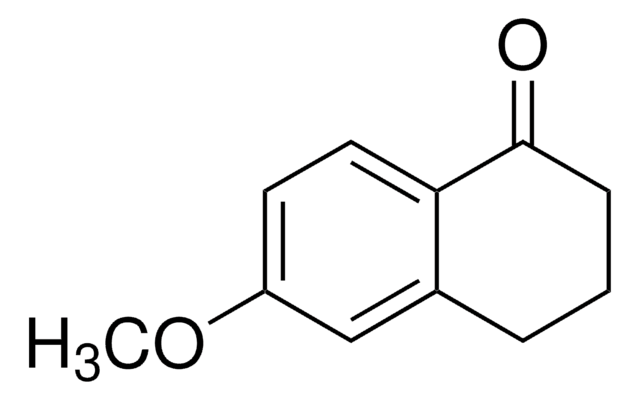
C12H14O3
Numer CAS:
Masa cząsteczkowa:
206.24
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Próba
97%
Postać
powder
mp
98-100 °C (lit.)
ciąg SMILES
COc1cc2CCCC(=O)c2cc1OC
InChI
1S/C12H14O3/c1-14-11-6-8-4-3-5-10(13)9(8)7-12(11)15-2/h6-7H,3-5H2,1-2H3
Klucz InChI
YNNJHKOXXBIJKK-UHFFFAOYSA-N
Opis ogólny
6,7-Dimethoxy-1-tetralone reacts with 2-amino-4,5-dimethoxyacetophenone to form 5,6-dihydro-2,3,9,10-tetramethoxybenz[c]acridine.
Zastosowanie
6,7-Dimethoxy-1-tetralone was used in the synthesis of 2-bromotetralones by undergoing bromination. It was also used as a precursor to quinolines with dopaminergic activity, naphthols with anti-inflammatory activity and benzophenanthridine alkaloids with antitumor activity.
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Colin J Dunsmore et al.
Bioorganic & medicinal chemistry letters, 18(5), 1730-1734 (2008-02-12)
Several 2-aminotetralones were identified as novel inhibitors of the bacterial enzymes MurA and MurZ. A number of these inhibitors demonstrated antibacterial activity against Staphylococcus aureus and Escherichia coli with MICs in the range 8-128 microg/ml. Based on structure-activity relationships we
D G Batt et al.
Journal of medicinal chemistry, 33(1), 360-370 (1990-01-01)
The synthesis, biological evaluation, and structure-activity relationships of a series of 1-naphthols bearing carbon substituents at the 2-position are described. These compounds are potent inhibitors of the 5-lipoxygenase from RBL-1 cells and also inhibit bovine seminal vesicle cyclooxygenase. Structure-activity relationships
The Journal of Organic Chemistry, 57, 5907-5907 (1992)
J C Craig et al.
Journal of medicinal chemistry, 32(5), 961-968 (1989-05-01)
A series of 2-substituted octahydrobenzo[f]quinolines has been synthesized and assayed for dopamine agonist activity. Only the compounds corresponding to the beta-rotameric conformation of dopamine showed biphasic activity in competition binding studies with the radioligand [3H]spiroperidol. These findings suggest that the
D Makhey et al.
Bioorganic & medicinal chemistry, 8(5), 1171-1182 (2000-07-06)
Coralyne and several other synthetic benzo[a,g]quinolizium derivatives related to protoberberine alkaloids have exhibited activity as topoisomerase poisons. These compounds are characterized by the presence of a positively charged iminium group, which has been postulated to be associated with their pharmacological
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej