Key Documents
182249
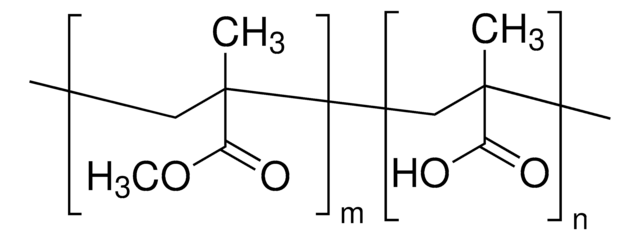
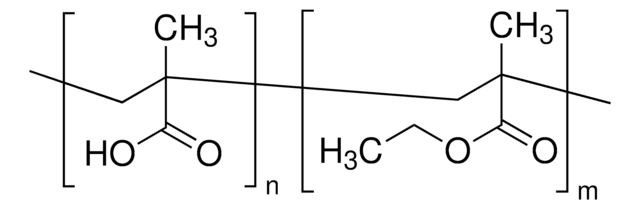
Poly(methyl methacrylate-co-ethyl acrylate)
ethyl acrylate <5 wt. %, average Mn ~39,500 by GPC, average Mw ~101,000 by GPC, powder
About This Item
Polecane produkty
Postać
powder
Poziom jakości
temp. samozapłonu
580 °F
masa cząsteczkowa
average Mn ~39,500 by GPC
average Mw ~101,000 by GPC
skład
ethyl acrylate, <5 wt. %
temp. przejścia
Tg (DSC) 104 °C (onset)
InChI
1S/2C5H8O2/c1-4(2)5(6)7-3;1-3-5(6)7-4-2/h1H2,2-3H3;3H,1,4H2,2H3
Klucz InChI
XPNLOZNCOBKRNJ-UHFFFAOYSA-N
Zastosowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Dr. Tan and researcher introduce recent trends in Self-healing Soft Electronic Materials and Devices. The emergence of smart, functional SHPs will be highly beneficial to the advancement of the next-generation self-healing soft electronic devices. Autonomously self-healing devices could help to minimize the need for repair or replacement of electronics and machines, potentially reducing the cost of materials and reducing electronic waste.
Self-assembled monolayers (SAMs) have attracted enormous interest for a wide variety of applications in micro- and nano-technology. In this article, we compare the benefits of three different classes of SAM systems (alkylthiolates on gold.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej