Wszystkie zdjęcia(1)
Key Documents
144908
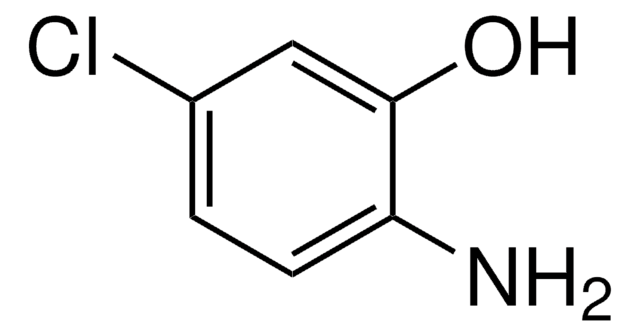
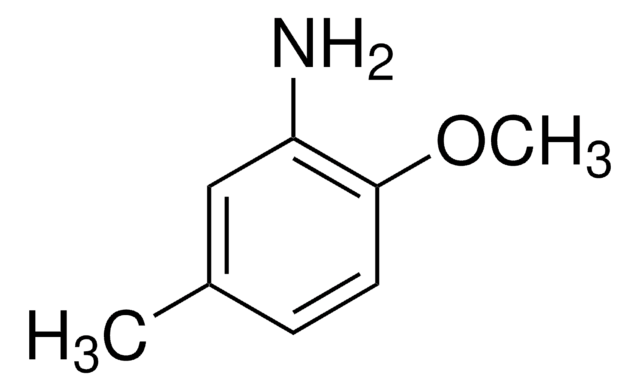
2-Amino-4-methylphenol
97%
Synonim(y):
2-Amino-p-cresol, 2-Hydroxy-5-methylaniline, 3-Amino-4-hydroxytoluene
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór liniowy:
H2NC6H3(CH3)OH
Numer CAS:
Masa cząsteczkowa:
123.15
Beilstein:
606494
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Próba
97%
Postać
solid
mp
133-136 °C (lit.)
ciąg SMILES
Cc1ccc(O)c(N)c1
InChI
1S/C7H9NO/c1-5-2-3-7(9)6(8)4-5/h2-4,9H,8H2,1H3
Klucz InChI
ZMXYNJXDULEQCK-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
2-Amino-4-methylphenol is the major sensitizer in contact allergy to Disperse Yellow 3. It reacts with acetylacetone in absolute ethanol to yield 4-(2-hydroxy-5-methylphenyl)imino-2-pentanone. It was converted to dihydrophenoxazinone by purified human hemoglobin.
Zastosowanie
2-Amino-4-methylphenol was used in the synthesis of novel functionalized spiropyran derivatives of 2H-1,3-benzoxazinone series.
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Hongmei Peng et al.
Inorganic chemistry, 47(21), 9828-9835 (2008-10-03)
The synthesis and reactivity of a series of sodium and rare-earth metal complexes stabilized by a dianionic N-aryloxo-functionalized beta-ketoiminate ligand were presented. The reaction of acetylacetone with 1 equiv of 2-amino-4-methylphenol in absolute ethanol gave the compound 4-(2-hydroxy-5-methylphenyl)imino-2-pentanone (LH2, 1)
Antony O Bulanov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 1146-1152 (2008-06-14)
Six novel functionalized spiropyran's derivatives of 2H-1,3-benzoxazinone series were synthesized by introducing the substituents with chelating ability into 2H-chromene part of the 8'-formyl-7'-hydroxy-3-methyl-4-oxo-3,4-dihydro-2H-1,3-benzoxazine-2-spiro-2'-[2H]-chromene (I) by condensation with 2-aminophenol, 2-amino-4-methylphenol, 2-amino-4-nitrophenol, 2-amino-1-methylbenzimidazole, 4-amino-4H-1,2,4-triazole, N-(4-aminophenyl)acetamide. (1)H NMR, UV/vis, IR spectroscopy combined with
Z He et al.
Applied and environmental microbiology, 66(7), 3010-3015 (2000-07-06)
In spite of the variety of initial reactions, the aerobic biodegradation of aromatic compounds generally yields dihydroxy intermediates for ring cleavage. Recent investigation of the degradation of nitroaromatic compounds revealed that some nitroaromatic compounds are initially converted to 2-aminophenol rather
A Tomoda et al.
Journal of biochemistry, 110(6), 1004-1007 (1991-12-01)
2-Amino-4-methylphenol was converted to a brownish yellow material by the lysates of human erythrocytes or purified human hemoglobin. The reaction proceeded oxidatively, coupled with the oxidation of hemoglobin. The major component of the brownish yellow material produced by oxidative condensation
M Akazawa et al.
The Tohoku journal of experimental medicine, 192(4), 301-312 (2001-04-05)
When human erythrocytes were incubated with o-aminophenol at pH 7.0 at 37 degrees C for 46 hours, intracellular oxyhemoglobin was completely oxidized to methemoglobin during the initial 6 hours, and methemoglobin formed was then reduced to oxyhemoglobin during the following
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej