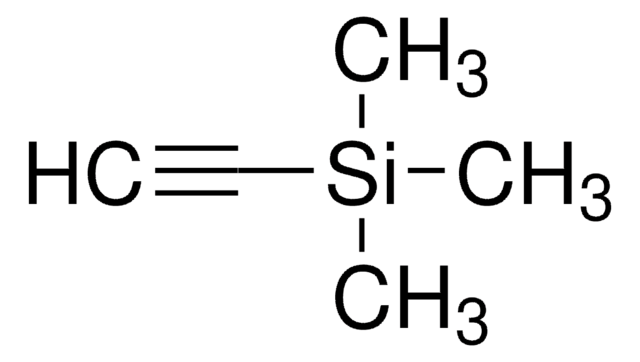모든 사진(1)
About This Item
Linear Formula:
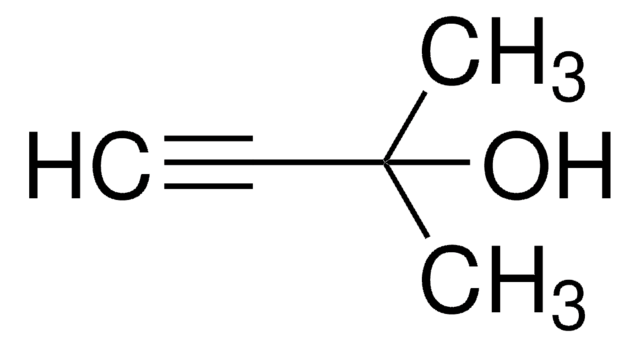
(CH3)3CSi(CH3)2C≡CH
CAS Number:
Molecular Weight:
140.30
Beilstein:
1921466
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
refractive index
n20/D 1.451 (lit.)
bp
116-117 °C (lit.)
density
0.751 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)C#C
InChI
1S/C8H16Si/c1-7-9(5,6)8(2,3)4/h1H,2-6H3
InChI key
RTYNRTUKJVYEIE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
(tert-Butyldimethylsilyl)acetylene is a bulky trialkylsilyl-protected alkyne. It participates in Cadiot-Chodkiewicz cross-coupling reaction with various bromoalkynes to afford synthetically useful unsymmetrical diynes.
(tert-Butyldimethylsilyl)acetylene readily undergoes cross-dimerization reaction with various internal phenyl acetylenes in the presence of rhodium dimers and bidentate phosphine ligands to afford enynes.
애플리케이션
(tert-Butyldimethylsilyl)acetylene may be used in the synthesis of β-alkynylketone and β-alkynyl aldehydes.
이미 열람한 고객
Asymmetric synthesis of beta-alkynyl aldehydes by rhodium-catalyzed conjugate alkynylation.
Takahiro Nishimura et al.
Angewandte Chemie (International ed. in English), 48(43), 8057-8059 (2009-09-22)
Trost BM and Li CJ.
Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (2014)
Joseph P Marino et al.
The Journal of organic chemistry, 67(19), 6841-6844 (2002-09-14)
Bulky trialkylsilyl-protected alkynes such as triethylsilyl (TES), tert-butyldimethylsilyl (TBS), and triisopropylsilyl (TIPS) acetylenes underwent the Cadiot-Chodkiewicz cross-coupling reaction with different bromoalkynes to form a variety of synthetically useful unsymmetrical diynes in good yields. The diyne alcohol 10 was transformed regio-
Steric tuning of silylacetylenes and chiral phosphine ligands for rhodium-catalyzed asymmetric conjugate alkynylation of enones.
Takahiro Nishimura et al.
Journal of the American Chemical Society, 130(5), 1576-1577 (2008-01-17)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.