추천 제품
vapor pressure
15 mmHg ( 20 °C)
Quality Level
분석
98%
형태
liquid
autoignition temp.
662 °F
expl. lim.
16.6 %
refractive index
n20/D 1.42 (lit.)
bp
104 °C (lit.)
mp
2.6 °C (lit.)
density
0.868 g/mL at 25 °C (lit.)
작용기
hydroxyl
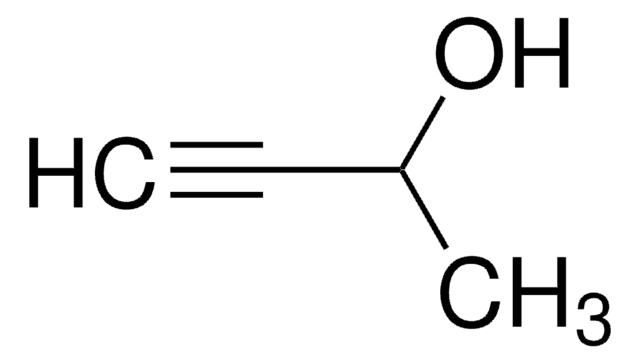
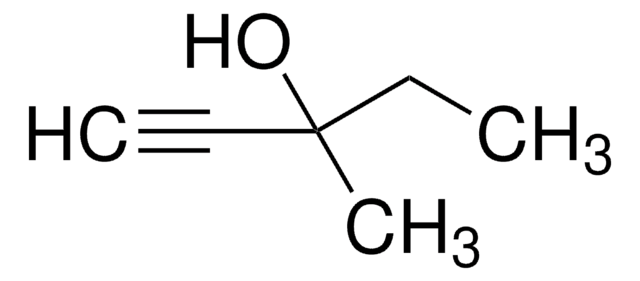
SMILES string
CC(C)(O)C#C
InChI
1S/C5H8O/c1-4-5(2,3)6/h1,6H,2-3H3
InChI key
CEBKHWWANWSNTI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Methyl-3-butyn-2-ol (MBY) is used as a precursor in the Mannich reaction and can undergo selective semihydrogenation to produce fine chemicals.
애플리케이션
2-Methyl-3-butyn-2-ol can be used as a reactant to synthesize:,·
- Aryl-2-methyl-3-butyn-2-ols via Pd-catalyzed Sonogashira coupling reaction with various aryl bromides.
- 2-Methyl-3-buten-2-ol (MBE) by Pd/γ-Al2O3 catalyzed selective hydrogenation reaction. MBE is applicable as an important intermediate in the synthesis of vitamin A.
- Diarylacetylenes via Pd-catalyzed Sonogashira coupling reaction with aryl chlorides in the presence of Cs2CO3 as a base.
- Optically active propargylic alcohols by enantioselective addition reaction with various aldehydes in the presence of Zn(OTf)2 and N-methylephedrine.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - STOT SE 3
표적 기관
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
66.2 °F - closed cup
Flash Point (°C)
19 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Boyall et al.
Organic letters, 2(26), 4233-4236 (2001-01-11)
We report the first example of enantioselective aldehyde additions of 2-methyl-3-butyn-2-ol, a commodity bulk chemical that is readily available. Following a facile fragmentation reaction, the addition reactions provide access to optically active terminal acetylenes as useful building blocks for asymmetric
Torstein Fjermestad et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(36), 10050-10057 (2011-07-21)
Density functional calculations were carried out to ascertain the origin of enantioselectivity in the brucine N-oxide (BNO)-assisted enantioselective Pauson-Khand reaction (PKR) of norbornene with 2-methyl-3-butyn-2-ol. The computed ee value in acetone is 68 % (R), which compares well to the previously
Micaela Crespo-Quesada et al.
Journal of the American Chemical Society, 133(32), 12787-12794 (2011-07-14)
The activity and selectivity of structure-sensitive reactions are strongly correlated with the shape and size of the nanocrystals present in a catalyst. This correlation can be exploited for rational catalyst design, especially if each type of surface atom displays a
Palladium-Catalyzed Efficient and One-Pot Synthesis of Diarylacetylenes from the Reaction of Aryl Chlorides with 2-Methyl-3-butyn-2-ol
Yi Chenyi, et al.
Advanced Synthesis & Catalysis, 349(10), 1738-1742 (2007)
Shinji Harada et al.
Chemical communications (Cambridge, England), (9)(9), 948-950 (2007-02-22)
Indium(III)-catalyzed asymmetric alkynylation of aryl, heteroaryl, alkyl and alkenyl aldehydes with 2-methyl-3-butyn-2-ol as an ethyne equivalent donor was realized, and products were obtained in moderate to good yields (up to 97%) and high enantioselectivities (up to 99% ee) using 2-10
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.