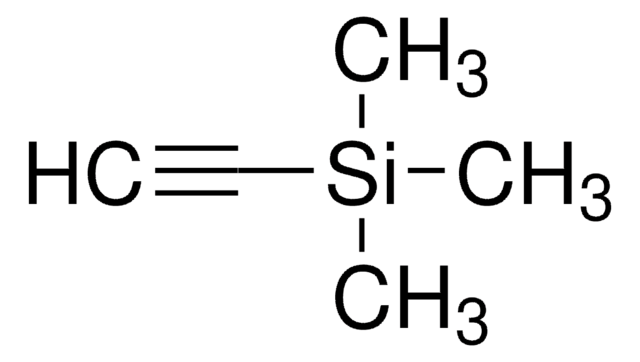About This Item
추천 제품
분석
99%
refractive index
n20/D 1.427 (lit.)
bp
136-137 °C (lit.)
mp
21-24 °C (lit.)
density
0.752 g/mL at 25 °C (lit.)
저장 온도
2-8°C
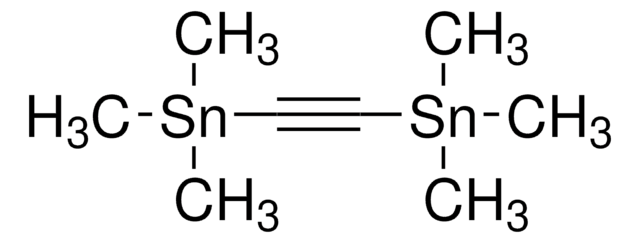
SMILES string
C[Si](C)(C)C#C[Si](C)(C)C
InChI
1S/C8H18Si2/c1-9(2,3)7-8-10(4,5)6/h1-6H3
InChI key
ZDWYFWIBTZJGOR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.