HPLC Analysis of Acetaminophen, Caffeine, Chlorpheniramine, Ibuprofen, Naproxen, Phenylephrine, and Pseudoephedrine on Ascentis® Express C18
材料
分析カラム
製品番号
詳細
価格
標準
製品番号
詳細
価格
CONDITIONS
column
Ascentis Express C18, 5 cm x 2.1 mm I.D., 2.7 μm particles (53822-U)
mobile phase
[A] 5 mM ammonium phosphate monobasic, pH 2.0 with phosphoric acid: acetonitrile (98:2); [B] acetonitrile;
gradient
0 to 68% B in 3 min; held at 68% B for 2 min
flow rate
0.4 mL/min
column temp.
35 °C
detector
UV, 210 nm
injection
1 μL
sample
100 μg/mL in 95:5, water: methanol
詳細
アナリシスノート
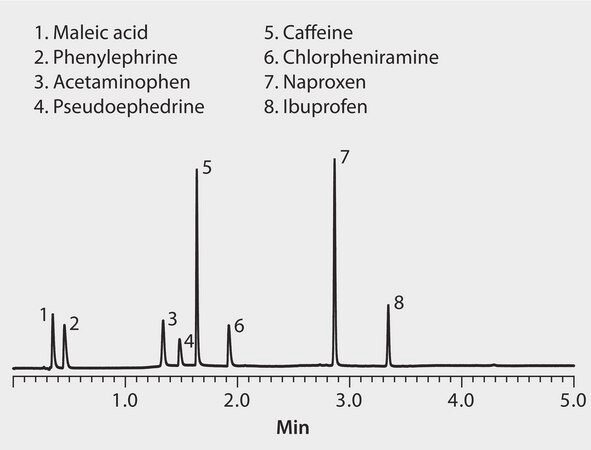
This application demonstrates the suitability of Ascentis Express C18 for the efficient separation of various over the counter drugs.
法的情報
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany