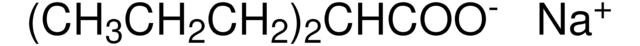おすすめの製品
品質水準
アッセイ
≥99% (HPLC)
形状
powder
mp
184-186 °C (lit.)
溶解性
H2O: 50 mg/mL
保管温度
−20°C
SMILES記法
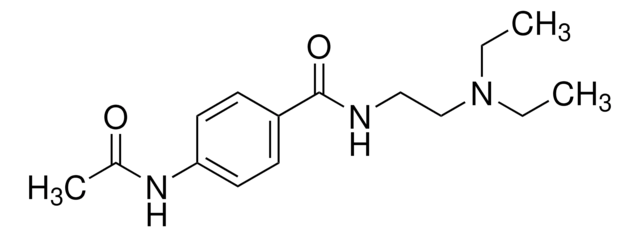
Cl[H].CCN(CC)CCNC(=O)c1ccc(NC(C)=O)cc1
InChI
1S/C15H23N3O2.ClH/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19;/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19);1H
InChI Key
IYEWBJUCJHKLHD-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
N-Acetylprocainamide hydrochloride may be used:
- as an internal standard for spiking plasma samples for ultra-high-pressure liquid chromatography coupled with a diode array detector (UHPLC-DAD) analysis
- to test its relaxant effect on tracheal smooth muscle tissue preparations
- in preparation of complexes with N-acetyl-L-tyrosine methyl ester and N-acetyl-L-phenylalanine methyl ester for studying intermolecular interactions using nuclear magnetic resonance (NMR) spectroscopy studies
N-Acetylprocainamide hydrochloride is a class III antiarrhythmic compound. N-Acetylprocainamide hydrochloride has been used in a study to determine the disposition of procainamide and N-acetylprocainamide in protein-calorie malnutrition. N-Acetylprocainamide hydrochloride has also been used to study pharmacokinetics of procainamide and N-acetylprocainamide in rats.
生物化学的/生理学的作用
N-acetyltransferase II in liver catalyzes the conversion of procainamide to N-acetylprocainamide (NAPA).
Class III antiarrhythmic. Increases the duration of the action potential by decreasing the delayed outward potassium current, slightly decreasing the calcium current, and slightly depressing the inward rectifier potassium current. This is the active metabolite of procainamide that does not induce systemic lupus erythematosus.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
A5513-BULK:
A5513-5G:
A5513-500MG:
A5513-1G:
A5513-VAR:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
B L Kamath et al.
Journal of pharmaceutical sciences, 70(3), 299-302 (1981-03-01)
The pharmacokinetics of distribution and elimination of procainamide and its major metabolite, N-actylprocainamide, were studied in rats. Eight rats were selected randomly, and each received intravenously 14C-labeled procainamide hydrochloride (75 mg/kg) or 14C-labeled N-acetylprocainamide hydrochloride (86 mg/kg) according to a
D Jung et al.
Drug metabolism and disposition: the biological fate of chemicals, 13(3), 359-363 (1985-05-01)
The influence of dietary protein deficiency on the disposition of procainamide (PA) and its major metabolite, N-acetylprocainamide (NAPA) was investigated in male Sprague-Dawley rats fed for 4 weeks on a 23 (control) or a 5% (low) protein diet ad libitum.
The evidence for complex formation between N-acetyl-l-tyrosine methyl ester and N-acetylprocainamide hydrochloride using NMR spectroscopy
Janik A, et al.
Structural Chemistry, 20(4), 699-707 (2009)
Larry A Bauer et al.
Antimicrobial agents and chemotherapy, 49(4), 1649-1651 (2005-03-29)
Ten healthy adults participated in a randomized, crossover drug interaction study testing procainamide only, procainamide plus levofloxacin, and procainamide plus ciprofloxacin. During levofloxacin therapy, most procainamide and N-acetylprocainamide (NAPA) pharmacokinetic parameters, including decreased renal clearances and renal clearance/creatinine clearance ratios
M Boucher et al.
Journal of autonomic pharmacology, 18(2), 83-87 (1998-09-08)
1. The cardiac anticholinergic effects of procainamide (1 mg kg(-1) min(-1)) and its N-acetylated metabolite (NAPA) at equimolar dose (1.16 mg kg(-1) min(-1)) were studied using in vivo experimental pharmacological and in vitro radioligand binding studies. 2. Procainamide and NAPA
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)