おすすめの製品
詳細
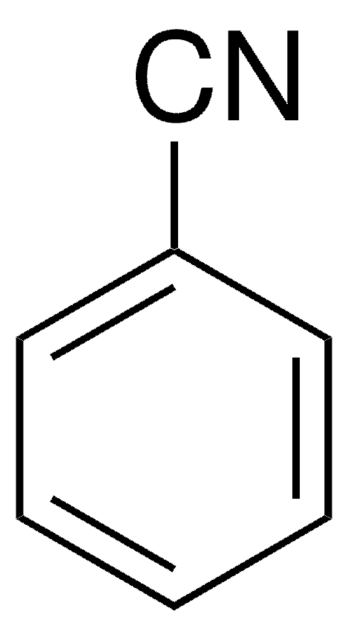
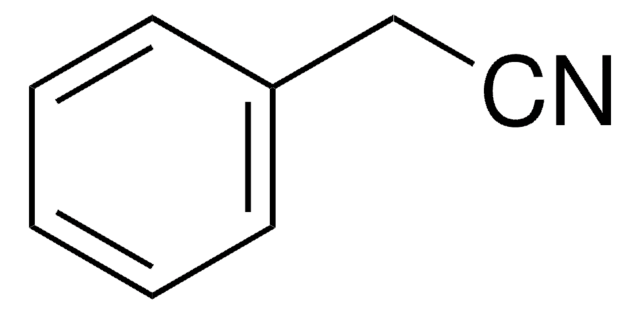
Benzonitrile, also known as a phenyl cyanide compound, is a useful solvent and a versatile precursor to many derivatives. It is a good solvent for the study of inorganic, organic, anhydrous, and organometallic compounds.
アプリケーション
Benzonitrile can be used as:
- An electrochemical solvent to investigate the electrochemistry, spectroscopic properties, and reactivity of a series of cobalt porphyrins with various substituents.
- Building block or starting material in various organic synthesis reactions.
- Employed in coupling reactions, such as Suzuki couplings or Heck reactions, to facilitate the formation of carbon-carbon bonds.
法的情報
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
保管分類コード
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
引火点(°F)
158.0 °F - closed cup
引火点(℃)
70 °C - closed cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
劇物
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
B8959-2.5L:
B8959-250ML:
B8959-VAR:
B8959-500ML:4548173189796
B8959-10L:
B8959-100ML:4548173189765
B8959-2L:4548173189789
B8959-18L:4548173276410
B8959-5ML:
B8959-BULK:
B8959-20L:4548173189772
B8959-1L:
この製品を見ている人はこちらもチェック
Jessica L Durham et al.
Inorganic chemistry, 51(14), 7825-7836 (2012-07-07)
The preparation of two new families of hexanuclear rhenium cluster complexes containing benzonitrile and phenyl-substituted tetrazolate ligands is described. Specifically, we report the preparation of a series of cluster complexes with the formula [Re(6)Se(8)(PEt(3))(5)L](2+) where L = benzonitrile, p-aminobenzonitrile, p-methoxybenzonitrile
Rocío López-Rodríguez et al.
The Journal of organic chemistry, 77(21), 9915-9920 (2012-10-18)
A mild procedure for the Ir(III)-catalyzed nitrogen-directed ortho borylation of aromatic N,N-dialkylhydrazones using pinacolborane as the boron source has been developed. The methodology relies on a modified, hemilabile N,N ligand built on a 4-N,N-dimethylaminopyridine unit that provides high reactivity while
Mohsen Sajadi et al.
Physical chemistry chemical physics : PCCP, 13(39), 17768-17774 (2011-09-03)
Time-dependent Stokes shifts (TDSS) were measured for diverse polarity probes in water, heavy water, methanol, and benzonitrile, by broadband fluorescence up-conversion with 85 fs time resolution. In water the spectral dynamics is solute-independent and quantitatively described by simple dielectric continuum
Yoshimitsu Hashimoto et al.
Organic & biomolecular chemistry, 10(30), 6003-6009 (2012-05-19)
A variety of highly functionalized polycyclic isoxazoles are prepared by a two-step protocol: (1) 1,3-dipolar cycloaddition of o,o'-disubstituted benzonitrile oxides to para-quinone mono-acetals, then (2) dehydrogenation. The cycloaddition proceeds in a regioselective manner, favouring the formation of the 4-acyl cycloadducts
Johanna Ungersboeck et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 70(11), 2615-2620 (2012-09-04)
The intention for the present study was to implement a microfluidic set-up for N-(11)C-methylations in a flow-through microreactor device with [(11)C]DASB as model-compound and [(11)C]CH(3)I and [(11)C]CH(3)OTf, respectively, as (11)C-methylation agents. Due to an observed "aging" effect of the (11)C-methylation
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)