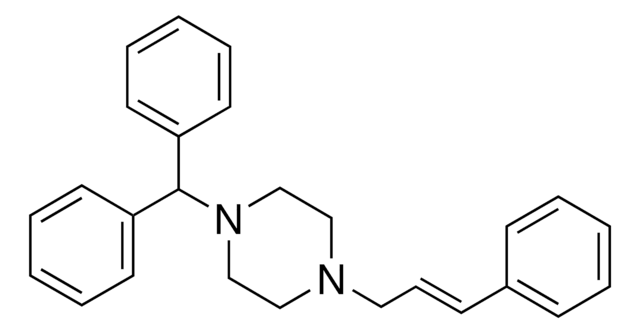
おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
cinnarizine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
C1CN(CCN1C\C=C\c2ccccc2)C(c3ccccc3)c4ccccc4
InChI
1S/C26H28N2/c1-4-11-23(12-5-1)13-10-18-27-19-21-28(22-20-27)26(24-14-6-2-7-15-24)25-16-8-3-9-17-25/h1-17,26H,18-22H2/b13-10+
InChI Key
DERZBLKQOCDDDZ-JLHYYAGUSA-N
遺伝子情報
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , HRH1(3269)
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Cinnarizine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Anne T Larsen et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 48(1-2), 339-350 (2012-11-28)
The in vivo performance of self-nanoemulsifying drug delivery systems (SNEDDSs) with different in vitro physicochemical properties were determined with the purpose of elucidating the parameters determining the in vivo performance of SNEDDSs. The in vitro characterisation included the use of
P Y Cezarino et al.
Climacteric : the journal of the International Menopause Society, 14(4), 492-496 (2011-03-24)
To evaluate the effectiveness and safety of cinnarizine in the treatment of menopausal symptoms. A total of 100 climacteric and symptomatic women participated in a double-blind, placebo-controlled study. They were divided into two groups of the same size: Gcin, intake
Mohamed A Alhnan et al.
International journal of pharmaceutics, 416(1), 55-60 (2011-06-18)
Poorly water soluble basic drugs are very sensitive to pH changes and following dissolution in the acidic stomach environment tend to precipitate upon gastric emptying, which leads to compromised or erratic oral bioavailability. In this work, we show that the
Amnon Gil et al.
Clinical neuropharmacology, 35(1), 37-39 (2011-12-06)
The objective of the study was to compare the efficacy of transdermal scopolamine and cinnarizine in the prevention of seasickness and their adverse reactions. Seventy-six naval crew members participated in a double-blind, randomized, crossover study. On 2 voyages, they were
Tri-Hung Nguyen et al.
Journal of controlled release : official journal of the Controlled Release Society, 153(2), 180-186 (2011-04-19)
This study is the first to demonstrate the ability of nanostructured liquid crystal particles to sustain the absorption of a poorly water soluble drug after oral administration. Cubic (V(2)) liquid crystalline nanostructured particles (cubosomes) formed from phytantriol (PHY) were shown
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)