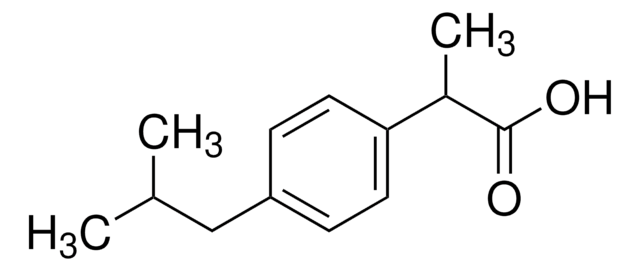
おすすめの製品
品質水準
アッセイ
≥98% (titration)
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
white
溶解性
ethanol: 1 mg/mL
DMSO: 5 mg/mL
輸送温度
ambient
保管温度
10-30°C
InChI
1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
InChI Key
HEFNNWSXXWATRW-UHFFFAOYSA-N
詳細
A nonsteroidal anti-inflammatory drug (NSAID) that acts as a reversible and competitive inhibitor of cyclooxygenase 1 (COX-1) (IC50 = 4.85 µM). Inhibits COX-2 at higher concentrations (IC50 = 223 µM). Blocks aspirin inactivation of COX-1 (EC50 antagonism of 200 µM aspirin = 290 nM). Shown to reduce the total Aβ secretion (Amyloid β40 and 42) in human neuronal cells and offers neuroprotection against glutamate-, nitric oxide-, and superoxide-induced damage. Reported to activate peroxisome proliferator-activated receptors (PPAR) α and γ in both CV-1 and C3H10T1/2 cells (~100 µM-500 µM).
A nonsteroidal anti-inflammatory drug (NSAID) that acts as a reversible, competitive, non-selective cyclooxygenase (COX) inhibitor (IC50 = 4.85 µM for purified COX-1 and 223 µM for purified COX-2). Also reported to inhibit COX-1 and COX-2 activity in intact bovine aortic endothelial cells (BAEC) (IC50 = 7 µM for COX-1 and 72.7 µM for COX-2). Potently blocks aspirin inactivation of COX-1 (EC50 antagonism of 200 µM aspirin ~290 nM for ovine COX-1). Decreases the secretion of total Aβ (Amyloid β40&42) by human neuronal cells and offers neuroprotection against glutamate-, nitric oxide- and superoxide-induced damage. Activates peroxisome proliferator activated receptors α and γ in both CV-1 and C3H10T1/2 cells (~100 µM - 500 µM).
生物化学的/生理学的作用
Cell permeable: no
Primary Target
COX-1
COX-1
Product competes with ATP.
Reversible: yes
Target IC50: 4.85 µM against COX-1
警告
Toxicity: Harmful (C)
再構成
Following reconstitution aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
その他情報
Asanuma, M., et al. 2001. J. Neurochem.76, 1895.
Blasko, I., et al. 2001. Neurobiol. Dis.8, 1094.
Ouellet, M., et al. 2001. Proc. Natl. Acad. Sci. USA98, 14583.
Casper, D., et al. 2000. Neurosci. Lett.289, 201.
Lambat, Z., et al. 2000. Metab. Brain Dis.15, 249.
Lim, G.P., et al. 2000. J. Neurosci.20, 5709.
Ogawa, O., et al. 2000. Eur. J. Pharmacol.408, 137.
Wyss-Coray, T., and Mucke, L. 2000. Nat. Med.6, 973.
Lehmann, J.M., et al. 1997. J. Biol. Chem.272, 3406.
Boneburg, E.M., et al. 1996. J. Clin. Pharmacol.36, 16S.
Mitchell, J.A., et al. 1994. Proc. Natl. Acad. Sci. USA90, 11693.
Blasko, I., et al. 2001. Neurobiol. Dis.8, 1094.
Ouellet, M., et al. 2001. Proc. Natl. Acad. Sci. USA98, 14583.
Casper, D., et al. 2000. Neurosci. Lett.289, 201.
Lambat, Z., et al. 2000. Metab. Brain Dis.15, 249.
Lim, G.P., et al. 2000. J. Neurosci.20, 5709.
Ogawa, O., et al. 2000. Eur. J. Pharmacol.408, 137.
Wyss-Coray, T., and Mucke, L. 2000. Nat. Med.6, 973.
Lehmann, J.M., et al. 1997. J. Biol. Chem.272, 3406.
Boneburg, E.M., et al. 1996. J. Clin. Pharmacol.36, 16S.
Mitchell, J.A., et al. 1994. Proc. Natl. Acad. Sci. USA90, 11693.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
401003-GM:
401003-1GM:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
M Ouellet et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(25), 14583-14588 (2001-11-22)
Both nonsteroidal anti-inflammatory drugs, such as ibuprofen, and the prototypical selective cyclooxygenase (Cox)-2 inhibitors DuP-697 and NS-398 block the inhibition of Cox-1 by aspirin in vitro. However, clinical studies have shown that the Cox-2 selective drugs (or coxibs) rofecoxib and
J A Mitchell et al.
Proceedings of the National Academy of Sciences of the United States of America, 90(24), 11693-11697 (1993-12-15)
Constitutive cyclooxygenase (COX-1; prostaglandin-endoperoxide synthase, EC 1.14.99.1) is present in cells under physiological conditions, whereas COX-2 is induced by some cytokines, mitogens, and endotoxin presumably in pathological conditions, such as inflammation. Therefore, we have assessed the relative inhibitory effects of
D Casper et al.
Neuroscience letters, 289(3), 201-204 (2000-08-29)
Non-steroidal anti-inflammatory drugs (NSAIDs) reduce the risk of Alzheimer's disease, although the underlying mechanisms are unknown. Glutamate excitotoxicity has been implicated in Alzheimer's disease, Parkinson's disease, and others. We examined the effects of aspirin, acetaminophen, and ibuprofen on cultured primary
E M Boneberg et al.
Journal of clinical pharmacology, 36(12 Suppl), 16S-19S (1996-12-01)
Since the discovery of a cytokine-inducible isozyme of cyclooxygenase (COX-2), its pharmacologic inhibition has been the subject of recent investigations. These include tests for the selectivity of known nonsteroidal antiinflammatory drugs (NSAIDs) on the constitutive enzyme of cyclooxygenase (COX-1) compared
Ibuprofen, inflammation and Alzheimer disease.
T Wyss-Coray et al.
Nature medicine, 6(9), 973-974 (2000-09-06)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)