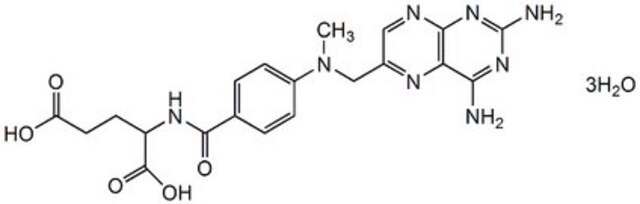
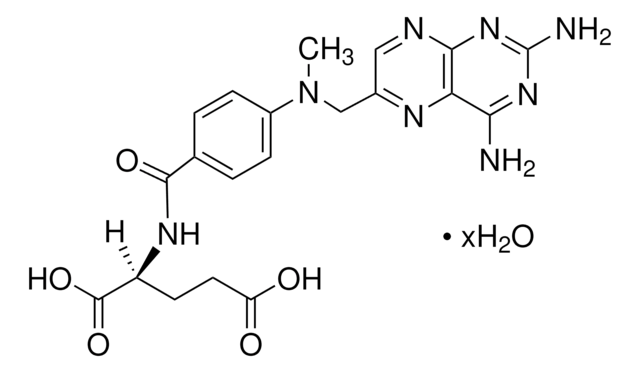
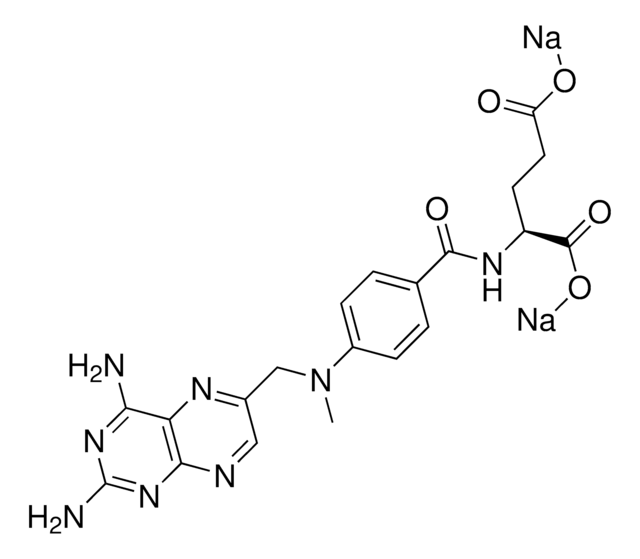
おすすめの製品
グレード
certified reference material
品質水準
形状
liquid
特徴
Snap-N-Spike®/Snap-N-Shoot®
包装
ampule of 1 mL
メーカー/製品名
Cerilliant®
濃度
1.0 mg/mL in methanol with 0.1N NaOH
テクニック
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
single component solution
保管温度
−20°C
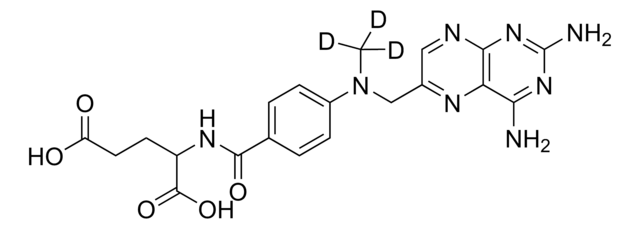
SMILES記法
O=C(C1=CC=C(N(C)CC2=NC3=C(N=C2)N=C(N)N=C3N)C=C1)NC(C(O)=O)CCC(O)=O
InChI
1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)
InChI Key
FBOZXECLQNJBKD-UHFFFAOYSA-N
詳細
アプリケーション
- Pharmacokinetic analysis: Methotrexate solution is employed in advanced pharmacokinetic studies using UV-Vis spectrophotometric and colorimetric methods. This application facilitates precise quantification in plasma and tissue, crucial for evaluating drug distribution and effectiveness in cancer treatments (Febrianti et al., 2024).
- Toxicity and safety monitoring: Observational studies utilize Methotrexate solution to investigate root causes of medication errors and manage toxicity in elderly patients. This research supports safer clinical practices by identifying risk factors and improving patient safety protocols (Bisht et al., 2024).
- Nephrotoxicity prevention: Research on Methotrexate solution examines hydration strategies to mitigate nephrotoxicity, enhancing therapeutic outcomes. This study is pivotal in optimizing Methotrexate use in clinical settings, ensuring higher safety and efficacy for patients undergoing chemotherapy (Hasanpour et al., 2024).
法的情報
シグナルワード
Danger
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - Met. Corr. 1 - STOT SE 1
保管分類コード
3 - Flammable liquids
WGK
WGK 2
引火点(°F)
51.8 °F - closed cup
引火点(℃)
11 °C - closed cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
アルコール類
危険等級II
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
M-136-1ML:4.548173318394E12
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)