おすすめの製品
フォーム
powder
包装
pkg of 1 × 1 mg (860657P-1mg)
pkg of 1 × 5 mg (860657P-5mg)
メーカー/製品名
Avanti Research™ - A Croda Brand 860657P
輸送温度
dry ice
保管温度
−20°C
SMILES記法
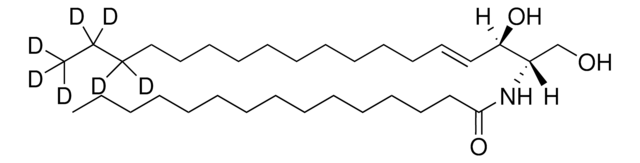
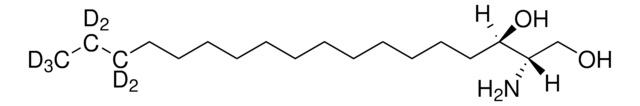
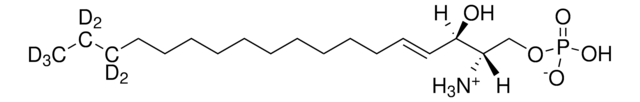
OC[C@@](N)([H])[C@@](O)([H])/C=C/CCCCCCCCCCC(C(C([2H])([2H])[2H])([2H])[2H])([2H])[2H]
InChI Key
WWUZIQQURGPMPG-AVMMVCTPSA-N
詳細
Sphingosine-d7 is a deuterated derivative of sphingosine. Sphingosine is an important bioactive sphingolipid metabolite. It is a 18-carbon amino alcohol derived from sphingomyelin. Sphingosine contains an unsaturated hydrocarbon chain.
アプリケーション
Sphingosine-d7 has been used as an internal standard in liquid chromatography–tandem mass spectrometry for quantitative analysis of sphingolipids in biological samples.
生物化学的/生理学的作用
Sphingosine negatively regulates cell proliferation and induces apoptosis. It has an ability to regulate the activities of phospholipases, protein kinases, ion channels, cannabinoid receptor type 1 (CB-1) receptors and steroidogenic factor 1 (SF-1) receptors. Sphingosine acts as a precursor for ceramide synthesis.
包装
5 mL Amber Glass Screw Cap Vial (860657P-1mg)
5 mL Amber Glass Screw Cap Vial (860657P-5mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
よく一緒に購入される製品
保管分類コード
11 - Combustible Solids
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
860657P-VAR:
860657P-5MG:
860657P-1MG:
860657P-BULK:
最新バージョンのいずれかを選択してください:
Henning Carstens et al.
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 53(6), 1015-1028 (2019-12-20)
Pulmonary infections with Pseudomonas aeruginosa (P. aeruginosa) or Staphylococcus aureus (S. aureus) are of utmost clinical relevance in patients with cystic fibrosis, chronic obstructive pulmonary disease, after trauma and burn, upon ventilation or in immuno-compromised patients. Many P. aeruginosa and
Stephanie Schwalm et al.
The American journal of pathology, 187(11), 2413-2429 (2017-08-16)
Kidney fibrosis is a hallmark of chronic kidney disease and leads to extracellular matrix accumulation, organ scarring, and loss of kidney function. In this study, we investigated the role of sphingosine kinase-2 (SPHK2) on the progression of tubular fibrosis by
Iulia Zoicas et al.
Cells, 9(5) (2020-05-24)
Human and murine studies identified the lysosomal enzyme acid sphingomyelinase (ASM) as a target for antidepressant therapy and revealed its role in the pathophysiology of major depression. In this study, we generated a mouse model with overexpression of Asm (Asm-tgfb)
Irina Alecu et al.
Journal of lipid research, 58(1), 60-71 (2016-11-23)
The 1-deoxysphingolipids (1-deoxySLs) are atypical sphingolipids (SLs) that are formed when serine palmitoyltransferase condenses palmitoyl-CoA with alanine instead of serine during SL synthesis. The 1-deoxySLs are toxic to neurons and pancreatic β-cells. Pathologically elevated 1-deoxySLs cause the inherited neuropathy, hereditary
Olivier Cuvillier
Biochimica et biophysica acta, 1585(2-3), 153-162 (2003-01-18)
The sphingolipid metabolites ceramide, sphingosine, and sphingosine 1-phosphate contribute to controlling cell proliferation and apoptosis. Ceramide and its catabolite sphingosine act as negative regulators of cell proliferation and promote apoptosis. Conversely, sphingosine 1-phosphate, formed by phosphorylation of sphingosine by a
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)