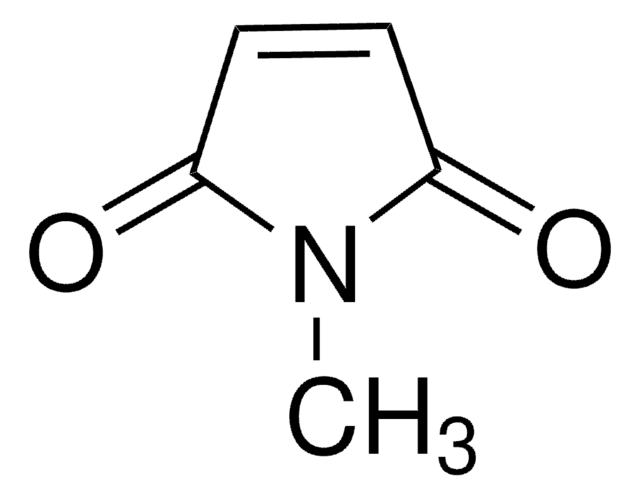
おすすめの製品
品質水準
アッセイ
97%
mp
89-91 °C (lit.)
官能基
imide
maleimide
SMILES記法
O=C1C=CC(=O)N1C2CCCCC2
InChI
1S/C10H13NO2/c12-9-6-7-10(13)11(9)8-4-2-1-3-5-8/h6-8H,1-5H2
InChI Key
BQTPKSBXMONSJI-UHFFFAOYSA-N
関連するカテゴリー
詳細
N-Cyclohexylmaleimide (CHMI, NCMI) is a cyclic imide. Diels-Alder reactions of 9-hydroxymethylanthracene with CHMI catalyzed by hydrophobic nanospace confined within the self-assembled Pd6 open cage bearing triimidazole walls have been reported. It undergoes Diels-Alder reactions with various aromatic hydrocarbons promoted by self-assembled coordination cage. This cage acts as nanometer-sized molecular flask and hence promotes this reaction. CHMI is reported to undergo radical polymerization readily under various polymerization conditions to afford poly(CHMI), having excellent thermal stability.
アプリケーション
N-Cyclohexylmaleimide (CHMI, NCMI) may be used for the preparation of hyperbranched copolymers of p-(chloromethyl)styrene (CMS) and NCMI, via atom transfer radical copolymerization reaction.
N-Cyclohexylmaleimide contaminated with N-cyclohexylmaleamic acid (less than 0.9 wt%) may be used for the heat-resistant poly(methyl methacrylate) resin free from coloration and for methyl methacrylate homopolymer. It may be used as monomer in the preparation of styrene/N-cyclohexylmaleimide copolymers with small polydispersities and controlled molecular weights, via free radical copolymerization. It may be used in the synthesis of hyperbranched copolymers by the atom transfer radical copolymerization with p-(chloromethyl)styrene catalyzed by CuCl/2,2′-bipyridine in cyclohexanone or anisole.
シグナルワード
Danger
危険有害性の分類
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1A - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
381543-1G:
381543-5G:
381543-25G:
381543-BULK:
381543-VAR:
この製品を見ている人はこちらもチェック
Shinnosuke Horiuchi et al.
Chemistry, an Asian journal, 6(7), 1839-1847 (2011-02-22)
A self-assembled coordination cage serves as a nanometer-sized molecular flask to promote the Diels-Alder reactions of aromatic hydrocarbons with N-cyclohexylmaleimide. The coordination cage accelerated the Diels-Alder reaction of anthracene at the electronically unfavorable, terminal benzene ring to give a compact
Increase in thermal stability of vinyl polymers through radical copolymerization with N-cyclohexylmaleimide.
Otsu T, et al.
Polymer International, 25(3), 179-184 (1991)
Living free radical donor-acceptor copolymerization of styrene and N-cyclohexylmaleimide and the synthesis of poly [styrene-co-(N-cyclohexylmaleimide)]/polystyrene block copolymers.
Schmidt-Naake G and Butz S.
Macromolecular Rapid Communications, 17(9), 661-665 (1996)
Bingchuan Wei et al.
Journal of chromatography. A, 1526, 104-111 (2017-10-29)
Reversed-phase liquid chromatography (RPLC) has been commonly used in IgG2 disulfide isoforms analysis. Recently, the columns packed with large pore superficially porous particles (SPP) have become available commercially. This work explores the application of this SPP technology in IgG2 disulfide
Hyperbranched copolymers of p-(chloromethyl) styrene and N-cyclohexylmaleimide synthesized by atom transfer radical polymerization.
Jiang X, et al.
Journal of Applied Polymer Science, 78(11), 1992-1997 (2000)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)