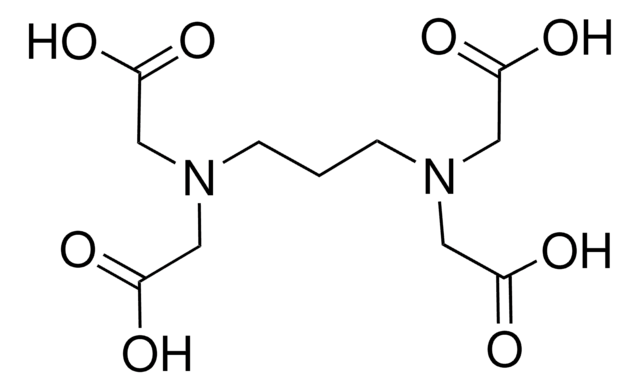
おすすめの製品
アッセイ
≥98%
フォーム
powder
mp
241 °C (dec.) (lit.)
官能基
amine
carboxylic acid
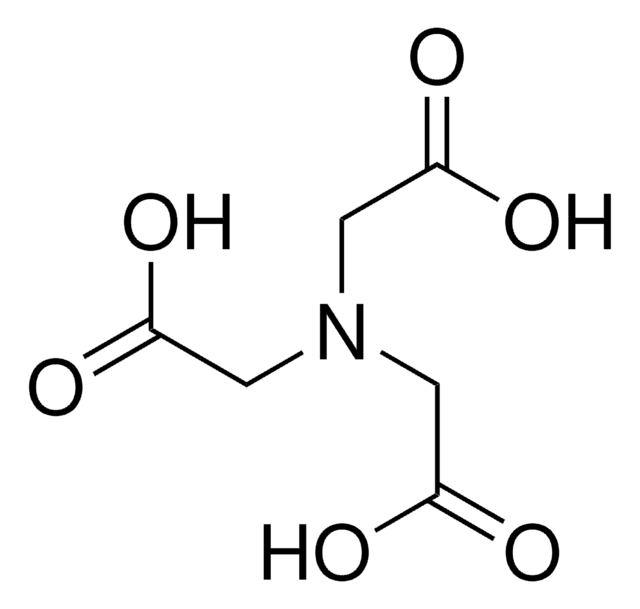
SMILES記法
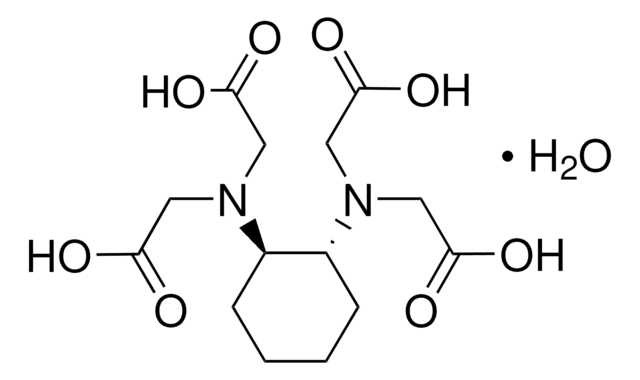
CC(CN(CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O
InChI
1S/C11H18N2O8/c1-7(13(5-10(18)19)6-11(20)21)2-12(3-8(14)15)4-9(16)17/h7H,2-6H2,1H3,(H,14,15)(H,16,17)(H,18,19)(H,20,21)
InChI Key
XNCSCQSQSGDGES-UHFFFAOYSA-N
アプリケーション
Employed in organometallic ligation.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
158135-BULK:
158135-10G:
158135-50G:
158135-VAR:
この製品を見ている人はこちらもチェック
Carsten K Schmidt et al.
Environmental pollution (Barking, Essex : 1987), 131(1), 107-124 (2004-06-24)
Aminopolycarboxylic acids, such as ethylenediaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA), diethylenetriaminepentaacetic acid (DTPA), 1,3-propylenediaminetetraacetic acid (1,3-PDTA), beta-alaninediacetic acid (beta-ADA), and methylglycinediacetic acid (MGDA), are used in large quantities in a broad range of industrial applications and domestic products in order
Bin Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(5), 1115-1121 (2010-10-12)
The eight-coordinate (enH2)[YIII(pdta)(H2O)](2)·10H2O (en=ethylenediamine and H4pdta=1,3-propylenediamine-N,N,N',N'-tetraacetic acid) was synthesized, meanwhile its molecular and crystal structures were determined by single-crystal X-ray diffraction technology. The interaction between [Y(III)(pdta)(H2O)]2(2-) and bovine serum albumin (BSA) was investigated by UV-vis and fluorescence spectra. The results
Journal of the Chemical Society. Chemical Communications, 381-381 (1993)
T P Ryan et al.
Chemical research in toxicology, 3(4), 384-390 (1990-07-01)
ADR-529 [(+)-1,2-bis(3,5-dioxopiperazin-1-yl)propane], a nonpolar, cyclic analogue of EDTA, protects against anthracycline cardiotoxicity in vivo. The protective mechanism presumably involves chelation of iron by a hydrolysis product of ADR-529, thus preventing the formation of reactive iron/oxygen species which can damage membrane
M Spiller et al.
Magnetic resonance in medicine, 8(3), 293-313 (1988-11-01)
The factors that determine the field-dependent increase in 1/T1 of tissue water protons were investigated for MnCl2 and Mn2+ (PDTA) (1,3-propylenediamine-N,N',N'',N'''-tetraacetic acid) introduced intravenously into rabbits. Mn2+ was used in preference to other paramagnetic ions in part because of the
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)