おすすめの製品
品質水準
アッセイ
98%
形状
solid
mp
>300 °C (lit.)
官能基
bromo
SMILES記法
Brc1ncnc2nc[nH]c12
InChI
1S/C5H3BrN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI Key
CTGFGRDVWBZYNB-UHFFFAOYSA-N
関連するカテゴリー
詳細
6-Bromopurine enhances the carcinostatic activity of azaserine in a test system employing ascites cell forms of sarcoma 180 and Ehrlich carcinoma in vivo. 6-bromopurine nucleosides are excellent substrates for substitution reactions with N-, O-, and S-containing nucleophiles in polar solvents.
アプリケーション
6-Bromopurine was used in the synthesis of 6-halopurine alkynes and corresponding triazole derivatives. 6-Bromopurine was used in the synthesis and chemical characterization of 2,3,4,5-tetrahydro-1,5-benzoxazepines-3-ol.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
104981-VAR:
104981-5G:
104981-BULK:
104981-1G:
Comparison of some biologgical and biochemical properties of 6-bromopurine and 6-iodopurine.
A C SARTORELLI et al.
Biochemical pharmacology, 11, 1017-1024 (1962-11-01)
E A Véliz et al.
The Journal of organic chemistry, 66(25), 8592-8598 (2001-12-12)
Surprisingly facile direct substitution reactions with acetyl-protected 6-bromopurine nucleosides are described. Included in the series of bromonucleosides studied is the guanosine derivative N(2)-2',3',5'-tetraacetyl-6-bromopurine ribonucleoside, the synthesis of which is reported here for the first time. Brominated nucleosides had not previously
Synthesis, unambiguous chemical characterization, and reactivity of 2, 3, 4, 5-tetrahydro-1, 5-benzoxazepines-3-ol.
Garcia-Rubino ME, et al.
Royal Society of Chemistry Advances, 2(33), 12631-12635 (2012)
Eva Galante et al.
Molecules (Basel, Switzerland), 18(5), 5335-5347 (2013-05-15)
2-[¹⁸F]Fluoroethyl azide ([¹⁸F]FEA) can readily be obtained by nucleophilic substitution of 2-azidoethyl-4-toluenesulfonate with [¹⁸F]fluoride (half-life 110 min), and has become widely used as a reagent for 'click' labeling of PET tracers. However, distillation of [18F]FEA is typically required, which is
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)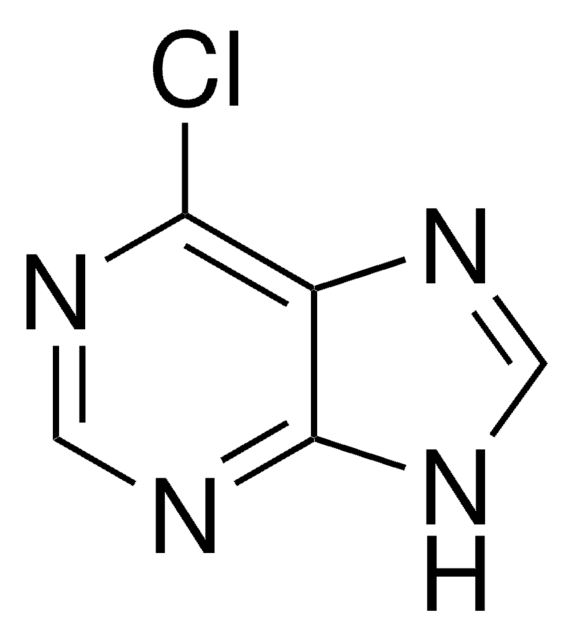
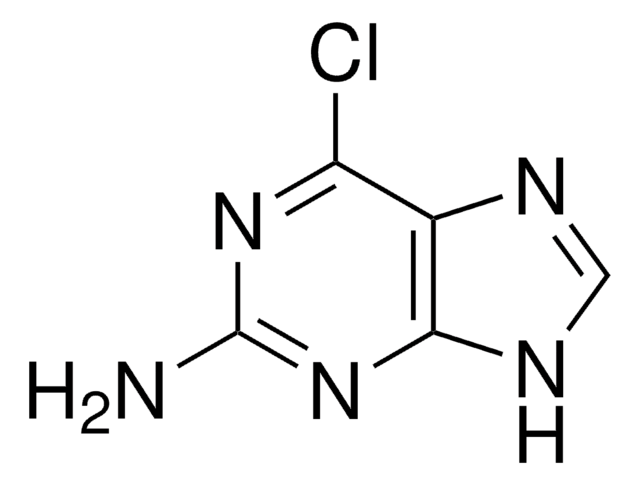
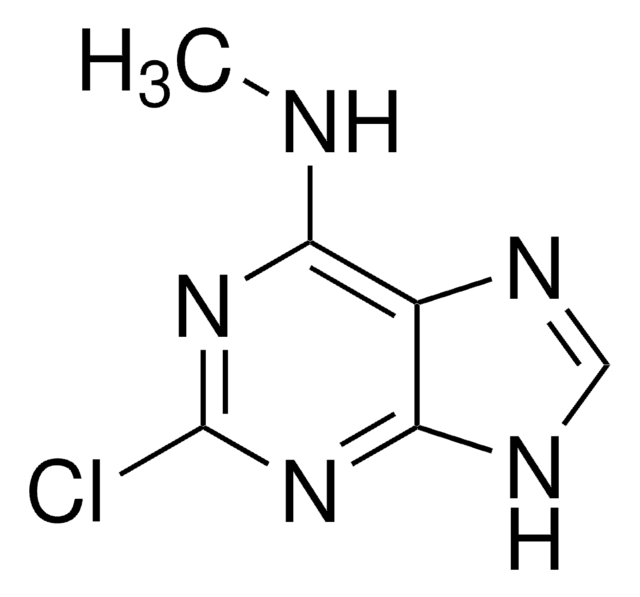
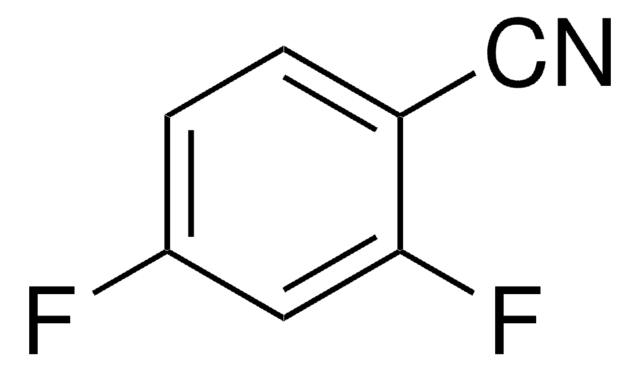
![2,4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine-7-carboxylic acid tert-butyl ester AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/315/036/b807f57a-439c-4114-b92d-077d429f82f3/640/b807f57a-439c-4114-b92d-077d429f82f3.png)
