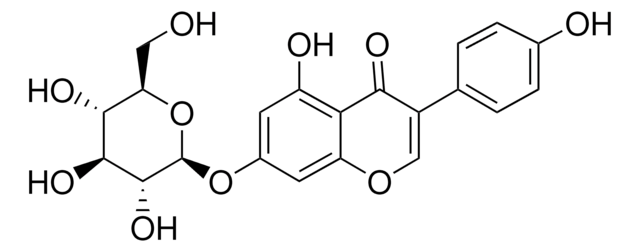
94334
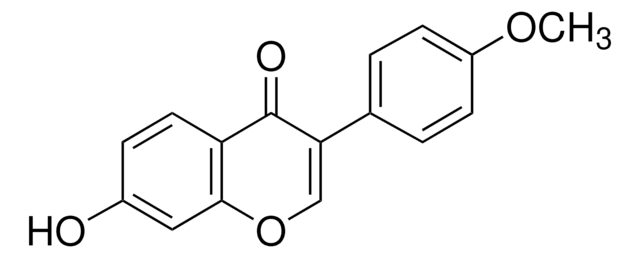
Formononetin
analytical standard
Sinonimo/i:
7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one, 7-Hydroxy-3-(4-methoxyphenyl)chromone, 7-Hydroxy-4′-methoxyisoflavone, Biochanin B, Daidzein 4′-methyl ether, Formonetin, Formononetol
About This Item
Prodotti consigliati
Grado
analytical standard
Saggio
≥98.0% (HPLC)
Durata
limited shelf life, expiry date on the label
applicazioni
food and beverages
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
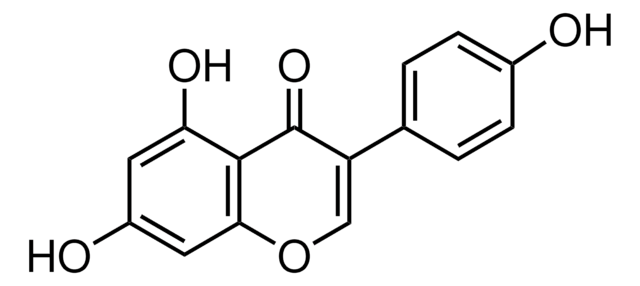
COc1ccc(cc1)C2=COc3cc(O)ccc3C2=O
InChI
1S/C16H12O4/c1-19-12-5-2-10(3-6-12)14-9-20-15-8-11(17)4-7-13(15)16(14)18/h2-9,17H,1H3
HKQYGTCOTHHOMP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Cow, goat, sheep and human milk samples by magnetic-micro-dispersive solid-phase extraction (m-μ-dSPE) combined with ultra-high performance liquid chromatography-triple quadrupole electrospray ionization-tandem mass spectrometry (UHPLC-QqQ-ESI-MS/MS) operating under the multiple reaction monitoring (MRM) mode of detection.
- Dairy products by quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction procedure and UHPLC-QqQ-ESI-MS/MS with MRM mode of detection.
It may be used as a reference standard for the determination of formononetin in:
- Natural products by turbulent flow chromatography liquid chromatography-high resolution mass spectrometry (TFC LC-HRMS).
- Soy-drinks by gas chromatography combined with MS/MS operating on electron ionization (EI) mode.
- Rat plasma by UHPLC-QqQ-ESI-MS/MS.
Azioni biochim/fisiol
Prodotti consigliati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.