47752
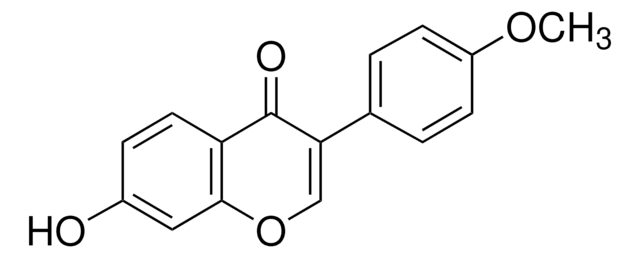
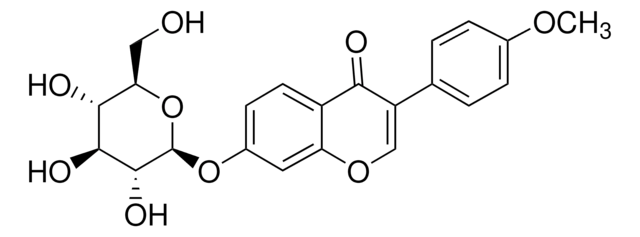
Formononetin
≥99.0% (TLC)
Sinonimo/i:
7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one, 7-Hydroxy-3-(4-methoxyphenyl)chromone, 7-Hydroxy-4′-methoxyisoflavone, Biochanin B, Daidzein 4′-methyl ether, Formonetin, Formononetol
About This Item
Prodotti consigliati
Saggio
≥99.0% (TLC)
Forma fisica
solid
Punto di fusione
257-261 °C
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Stringa SMILE
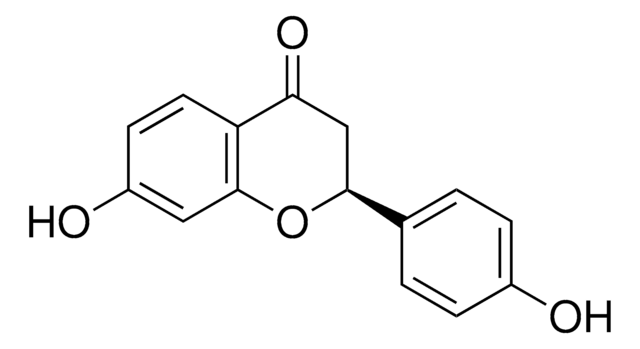
COc1ccc(cc1)C2=COc3cc(O)ccc3C2=O
InChI
1S/C16H12O4/c1-19-12-5-2-10(3-6-12)14-9-20-15-8-11(17)4-7-13(15)16(14)18/h2-9,17H,1H3
HKQYGTCOTHHOMP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as a reference standard to study export of multiple metabolites from Crocus sativus stigma extracts using liquid chromatography-photodiode array-high resolution mass spectrometry (LC-PDA-HRMS)
- as phytoestrogen-mimetic isoflavones to test its antioxidant effects in a biomimetic environment using chemiluminescence method
- as an authentic standard to test the isoflavones content level in both wild type and transgenic Medicago truncatula plant using high-performance liquid chromatography (HPLC)
- to test its effects as a potential Aryl hydrocarbon receptor (AhR)-agonist on the restoration of skin barrier proteins for Atopic dermatitis (AD) treatment using different keratinocyte cell lines and human skin equivalent (HSE) model
Azioni biochim/fisiol
Confezionamento
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.