914436
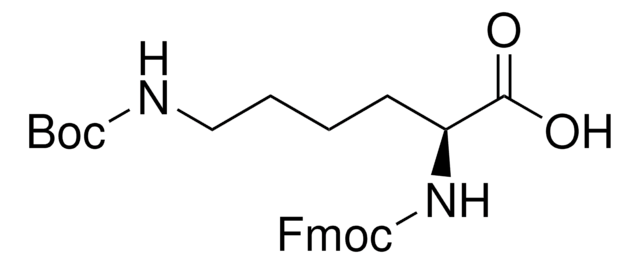
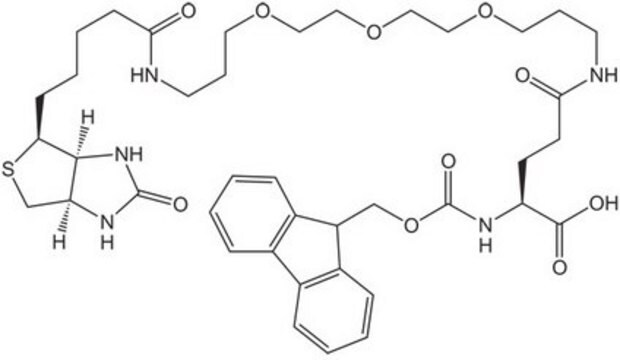
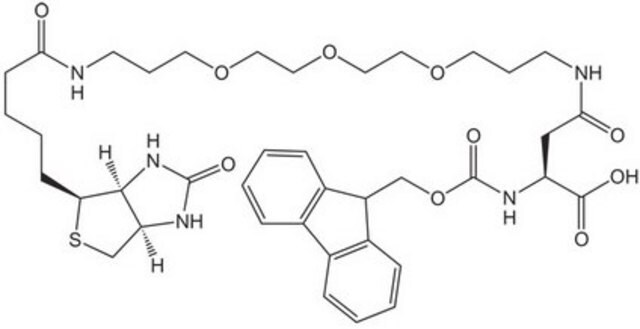
Fmoc-D-Lys(Biotin)-OH
≥95%
Sinonimo/i:
Nα-(9-Fluorenylmethyloxycarbonyl)-Nε-biotinyl-D-lysine, N2-(((9H-Fluoren-9-yl)methoxy)carbonyl)-N6-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanoyl)-D-lysine, Biotinylated Fmoc-protected D-lysine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Stato
powder
Punto di fusione
184-190 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
S1[C@H]([C@H]5NC(=O)N[C@H]5C1)CCCCC(=O)NCCCC[C@@H](NC(=O)OCC2c3c(cccc3)c4c2cccc4)C(=O)O
InChI
1S/C31H38N4O6S/c36-27(15-6-5-14-26-28-25(18-42-26)33-30(39)35-28)32-16-8-7-13-24(29(37)38)34-31(40)41-17-23-21-11-3-1-9-19(21)20-10-2-4-12-22(20)23/h1-4,9-12,23-26,28H,5-8,13-18H2,(H,32,36)(H,34,40)(H,37,38)(H2,33,35,39)/t24-,25+,26+,28+/m1/s1
OFIBQNGDYNGUEZ-KAHNYGAISA-N
Applicazioni
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 914436-100MG | 4061842093489 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.