481122
4-Ethynylaniline
97%
Sinonimo/i:
1-Amino-4-ethynylbenzene, P-APAC
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
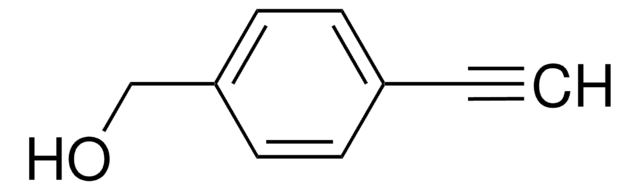
HC≡CC6H4NH2
Numero CAS:
Peso molecolare:
117.15
Beilstein:
2205181
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Impiego in reazioni chimiche
reaction type: click chemistry
Punto di fusione
98-102 °C (dec.) (lit.)
Stringa SMILE
Nc1ccc(cc1)C#C
InChI
1S/C8H7N/c1-2-7-3-5-8(9)6-4-7/h1,3-6H,9H2
JXYITCJMBRETQX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
4-Ethynylaniline, also known as p-ethynylaniline, is a terminal alkyne. Its synthesis using 2-methyl-3-butyn-2-ol (MEBYNOL) has been reported. The transition metal catalyzed polymerization of 4-ethynylaniline to afford poly(4-ethynylaniline) has been reported. The impact of the surface functionalization with 4-ethynylaniline on the thermal behavior of multi-walled carbon nanotubes (MWNTs) and graphene has been investigated.
Applicazioni
4-Ethynylaniline may be used in the synthesis of N-methyliminodiethyl 4-(4-ethynylphenyliminomethyl)benzeneboronate. It can also be used to prepare an acetylene ligand, HC2-NDI (NDI= 1,4,5,8-naphthalenediimide).
Used as an alkyne component in a synthesis of indoles from nitroarenes in the presence of a palladium-phenantroline catalyst.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Fabio Ragaini et al.
The Journal of organic chemistry, 71(10), 3748-3753 (2006-05-06)
Palladium-phenanthroline complexes efficiently catalyze the reaction of nitroarenes with arylalkynes and CO to give 3-arylindoles by an ortho-C-H functionalization of the nitroarene ring. Both electron-withdrawing and electron-donating substituents are tolerated on the nitroarene, except for bromide and activated chloride. Nitroarenes
Platinum (II) phosphine complexes with acetylene ligands containing 1,4,5,8-naphthalenediimide: Synthesis, crystal structure and electrochemistry.
Shavaleev NM, et al.
Journal of Organometallic Chemistry, 692(4), 921-925 (2007)
Comparative study of the covalent diazotization of graphene and carbon nanotubes using thermogravimetric and spectroscopic techniques.
Castelain M, et al.
Physical Chemistry Chemical Physics, 15(39), 16806-16811 (2013)
Arijit Sengupta et al.
International journal of biological macromolecules, 82, 256-263 (2015-10-18)
Amphiphilic hybrid graft copolymers were synthesized using a graft-to methodology and their protein adsorption profiles studied. Three different hydrophilic side chains were studied: hydroxypropylated high amylose starch, maltodextrin, and polyethylene glycol (PEG). In the high amylose starch compositions, there was
A simple and economical synthetic route to p-ethynylaniline and ethynyl-terminated substrates.
Melissaris AP and Litt MH.
The Journal of Organic Chemistry, 59(19), 5818-5821 (1994)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 481122-5G | 4061832388533 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.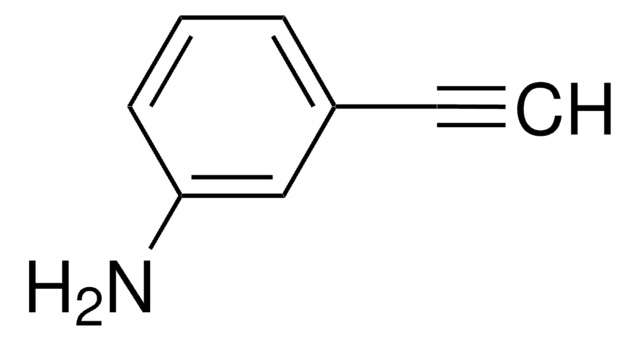


![4-[(4-Fluorophenyl)ethynyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/188/684/0b16c024-0d26-4b43-a607-60b40446e593/640/0b16c024-0d26-4b43-a607-60b40446e593.png)