206490
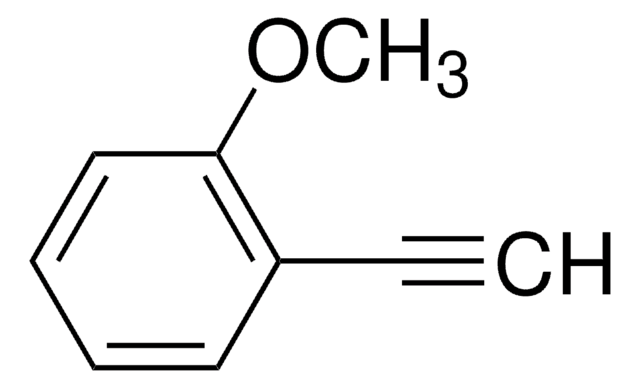
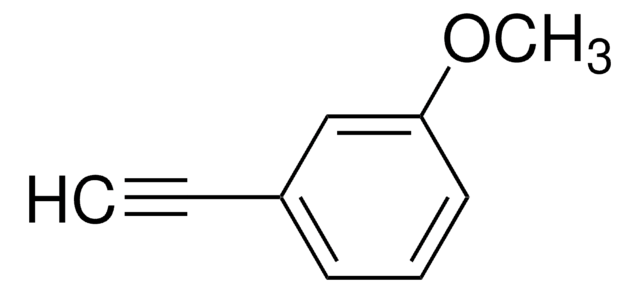
4-Ethynylanisole
97%
Sinonimo/i:
1-Ethynyl-4-methoxybenzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3OC6H4C≡CH
Numero CAS:
Peso molecolare:
132.16
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
Indice di rifrazione
n20/D 1.563 (lit.)
P. ebollizione
87-91 °C/11 mmHg (lit.)
Punto di fusione
28-29 °C (lit.)
Densità
1.019 g/mL at 25 °C (lit.)
Stringa SMILE
COc1ccc(cc1)C#C
InChI
1S/C9H8O/c1-3-8-4-6-9(10-2)7-5-8/h1,4-7H,2H3
KBIAVTUACPKPFJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
4-Ethynylanisole forms the corresponding propargyl aldehyde in good yield directly from DMF-dimethyl acetal without the need for making an acetylide salt. 1,3-dipolar cycloaddition of acceptor-cyclopropylmethylsilanes affords functionalized cyclopentenes in good yields.
4-Ethynylanisole can be used as a building block in organic synthesis and also used in sonogashira coupling reactions.
4-Ethynylanisole can be used as a building block in organic synthesis and also used in sonogashira coupling reactions.
Applicazioni
1,3-dipolar cycloaddition of acceptor-cyclopropylmethylsilanes affords functionalized cyclopentenes in good yields.
4-Ethynylanisole was used:
- in the synthesis of photoluminescent 1,2-dihydrophosphinines via a [4 + 2] cycloaddition
- along with an arylboronic acid and sodium azide in a copper-catalyzed, three-component synthesis of trisubstituted 1,2,4-triazoles
- in a study of a gold (III)-catalyzed hydroamination of alkynes leading to N-vinylindoles.
Forms the corresponding propargyl aldehyde in good yield directly from DMF-dimethyl acetal without the need for making an acetylide salt.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
179.6 °F - closed cup
Punto d’infiammabilità (°C)
82 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Synlett, 278-278 (2007)
Formal [3+2] addition of acceptor-substituted cyclopropylmethylsilanes with aryl acetylenes.
Veejendra K Yadav et al.
Angewandte Chemie (International ed. in English), 43(20), 2669-2671 (2008-07-17)
Laura C Pavelka et al.
Dalton transactions (Cambridge, England : 2003), 41(11), 3294-3301 (2012-01-31)
The addition of 4-trifluoromethyl-1-ethynylbenzene, phenylacetylene, or 4-ethynylanisole to P-mesityldiphenylmethylenephosphine, 1, produced photoluminescent 1,2-dihydrophosphinines 4a-c, respectively, in quantitative yield via a [4 + 2] cycloaddition. Limited reactivity was observed between 1 and non-aromatic alkynes. P-[Bis(trimethylsilyl)amino][(trimethylsilyl)methylene]phosphine, 2, and P-mesityl[(t-butyl)(trimethylsiloxy)methylene]phosphine, 3, showed extremely
Tetrahedron Letters, 45, 5043-5043 (2004)
Yuhua Zhang et al.
Organic letters, 9(4), 627-630 (2007-02-09)
A highly efficient double-hydroamination reaction of o-alkynylanilines with terminal alkynes leading to N-alkenylindoles was developed by using gold(III) as a catalyst under neat conditions. [reaction: see text].
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 206490-1G | 4061838768667 |
| 206490-250MG | |
| 206490-5G | 4061838768674 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.