186341
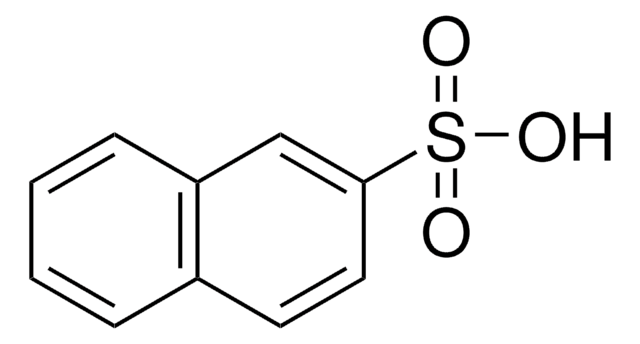
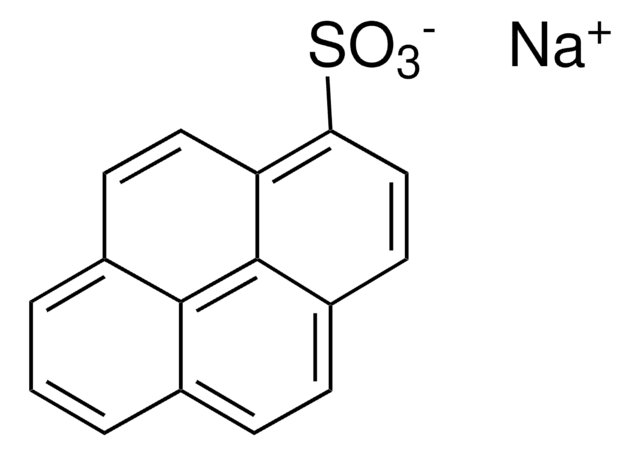
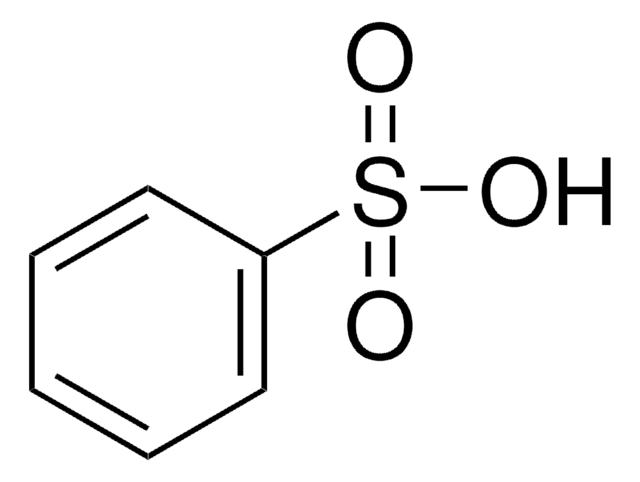
1-Naphthalenesulfonic acid
>50%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C10H7SO3H
Numero CAS:
Peso molecolare:
208.23
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Stato
solid
Livello qualitativo
Concentrazione
>50%
Punto di fusione
77-79 °C (lit.)
Solubilità
alcohol: freely soluble
diethyl ether: slightly soluble
water: freely soluble
Stringa SMILE
OS(=O)(=O)c1cccc2ccccc12
InChI
1S/C10H8O3S/c11-14(12,13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,11,12,13)
PSZYNBSKGUBXEH-UHFFFAOYSA-N
Informazioni sul gene
human ... EGFR(1956) , LCK(3932)
Descrizione generale
Mechanism of metabolism of 1-naphthalenesulfonic acid by green algae Scenedesmus obliquus has been investigated.
Applicazioni
1-Naphthalenesulfonic acid was used as template molecule to prepare new non-covalent molecularly imprinted polymer for solid-phase extraction of naphthalene sulfonates.
Altre note
remainder naphthalenesulfonic acid, sulfuric acid and water
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Reddicherla Umapathi et al.
Colloids and surfaces. B, Biointerfaces, 135, 588-595 (2015-09-01)
A lack of sufficient knowledge regarding the behaviour of stimuli-responsive polymers to biological stimuli hinders the potential use of responsive polymers as biomaterials and medical devices. Hence, in this study, we demonstrate the impact of various globular proteins on the
Jiao Guan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 202, 1-12 (2018-05-20)
The antimicrobial triclocarban (TCC) is frequently found in various personal care products (PCPs), and recent studies have demonstrated that it shows a high unintended biological activity on humans and wildlife. To evaluate the toxicity of TCC at the protein level
Yan Gong et al.
International journal of biological macromolecules, 101, 32-39 (2017-03-23)
The α-glucosidase inhibitor is of interest to researchers due to its association with type-2 diabetes treatment. Hesperetin is a flavonoid with natural antioxidant properties. This paper presents an evaluation on the effects of hesperetin on α-glucosidase via inhibitory kinetics using
Haibin Luo et al.
Journal of chromatography. A, 1424, 92-101 (2015-11-26)
We have systemically investigated unusual elution behaviors of an IgG4 (mAb A) in cation exchange chromatography (CEX). This mAb A exhibited two elution peaks under certain conditions when being purified by several strong CEX columns. When either of the two
Ester Caro et al.
Journal of chromatography. A, 1047(2), 175-180 (2004-10-06)
A new polymeric sorbent synthesised by exploiting molecular imprinting technology has been used to selectively extract naphthalene sulfonates (NSs) directly from aqueous samples. In the non-covalent molecular imprinting approach used to prepare this polymer, 1-naphthalene sulfonic acid (1-NS) and 4-vinylpyridine
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S21548-1EA | |
| 186341-1KG | |
| 186341-250G | |
| 186341-100G | |
| 186341-25G | 4061838758125 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.