L2140
Lectin from Bandeiraea simplicifolia (Griffonia simplicifolia)
Isolectin B4 (BSI-B4), biotin conjugate, lyophilized powder
Sinonimo/i:
Bandeirea simplicifolia agglutinin, BS-I
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
12352202
NACRES:
NA.32
Prodotti consigliati
Origine biologica
Bandieraea simplicifolia
Livello qualitativo
Coniugato
biotin conjugate
Stato
lyophilized powder
Potenza
<50 μg per mL agglutination activity (using human blood group B erythrocytes)
Composizione
Protein, ~95% E1%/280
Grado di funzionalizzazione
3-6 mol biotin per mol protein
Temperatura di conservazione
−20°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
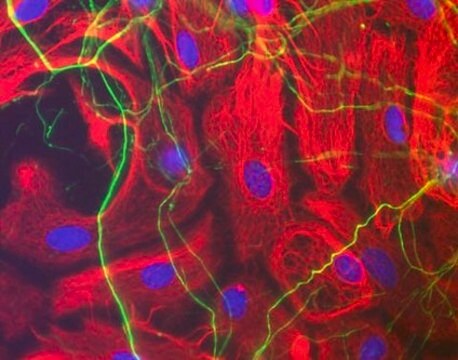
Lectin from Bandeiraea simplicifolia (Griffonia simplicifolia) has A and B subunits, which recognizes αGalNAc and αGal-end groups respectively. Lectins are extracted from the seeds of the legume shrub Griffonia simplicifolia, which are carbohydrate binding proteins.
Applicazioni
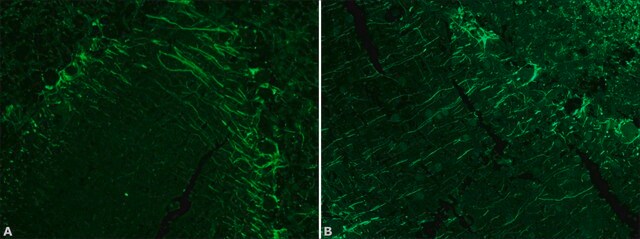
Lectin from Bandeiraea simplicifolia (Griffonia simplicifolia) has been used for immunohistochemistry and immunofluorescence staining.
Azioni biochim/fisiol
BS-I has a major affinity for terminal α-D-galactosyl residues with a secondary affinity for terminal N-acetyl-α-D-galactosaminyl residues.
Lectin from Bandeiraea simplicifolia (Griffonia simplicifolia) agglutinates human blood group A and B cells. It is specific for αGalNAc- and αGal. It acts as a reagent for the detection of this αGal epitope in biological materials. Lectin from Bandeiraea simplicifolia is used as a marker for xenoreactive antigen (αGal1−3Gal) and cancer. Lectin plays an important role in fertilization, embryogenesis, inflammation, metastasis and host-parasite recognition.
Altre note
BS-I is a tetrameric lectin consisting of two types of subunits designated A and B. There are five BS-I isolectins with different subunit composition: BSI-B4, BSI-AB3, BSI-A2B2, BSI-A3B and BSI-A4. BSI-B4 is blood group B specific and has an exclusive affinity for terminal α-D-galactosyl residues, whereas BSI-A4 has blood group A specificity and has a major affinity for terminal N-acetyl-α-D-galactosaminyl residues.
Stato fisico
Contains citrate buffer salts and calcium chloride
Risultati analitici
Agglutination activity is expressed in μg/mL and is determined from serial dilutions of a 1 mg/mL solution using phosphate buffered saline, pH 6.8, containing, for each lectin, calcium, magnesium, and manganese at different concentrations. This activity is the lowest concentration to agglutinate a 2% suspension of appropriate erythrocytes after 1 hr incubation at 25 °C.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Sensory processing of deep tissue nociception in the rat spinal cord and thalamic ventrobasal complex
Sikandar S, et al.
Physiological Reports, 5(14), e13323-e13323 (2017)
Isolectins IA and IB of Griffonia (Bandeiraea) simplicifolia crystal structure of metal-free gs i-b4 and molecular basis for metal binding and monosaccharide specificity
Lescar J, et al.
The Journal of Biological Chemistry, 277(8), 6608-6614 (2002)
Quan Yuan et al.
Tissue engineering. Part A, 24(9-10), 719-728 (2017-10-06)
The aim of this study was to analyze the three-dimensional distribution of hypoxia in the arteriovenous (AV) loop model in rats, by examining the distribution of hypoxia-inducible factor-1 alpha (HIF-1α). AV loops were created from the femoral artery and vein
Ludovic Wrobel et al.
Neuroscience letters, 495(1), 49-54 (2011-03-23)
Oxytocin can influence various spinal functions. However, little is known about the spinal neuronal networks responsible for oxytocin effects. The aim of this study was to localize and characterize spinal neurons expressing oxytocin receptors. We used an oxytocin receptor-reporter mouse
The Lectins: Properties, Functions, and Applications in Biology and Medicine (2012)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.