D7253
Dihydrostreptomycin sesquisulfate
Sinonimo/i:
Didromycin, Dihydrostreptomycin 3/2 sulfate, Dihydrostreptomycin sulfate
About This Item
Prodotti consigliati
Origine biologica
microbial
Forma fisica
powder
Colore
white to off-white
Spettro attività antibiotica
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
Modalità d’azione
protein synthesis | interferes
Temperatura di conservazione
2-8°C
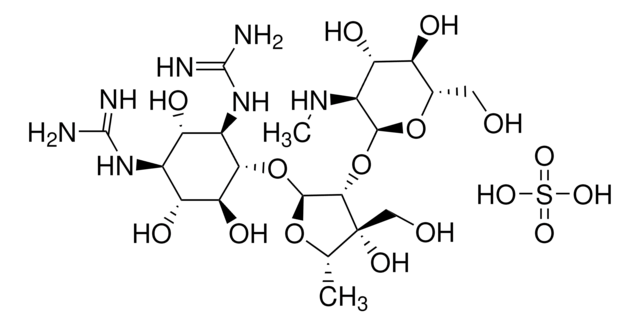
Stringa SMILE
OS(O)(=O)=O.OS(O)(=O)=O.OS(O)(=O)=O.CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@H]2[C@@H](O[C@@H](C)[C@]2(O)CO)O[C@@H]3[C@@H](O)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]3NC(N)=N.CN[C@H]4[C@H](O)[C@@H](O)[C@H](CO)O[C@H]4O[C@H]5[C@@H](O[C@@H](C)[C@]5(O)CO)O[C@@H]6[C@@H](O)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]6NC(N)=N
InChI
1S/2C21H41N7O12.3H2O4S/c2*1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35;3*1-5(2,3)4/h2*5-18,26,29-36H,3-4H2,1-2H3,(H4,22,23,27)(H4,24,25,28);3*(H2,1,2,3,4)/t2*5-,6-,7-,8+,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,21+;;;/m00.../s1
CZWJCQXZZJHHRH-YZTFXSNBSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Repr. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Extraction and quantitative analysis of aminoglycosides in porcine tissue, using molecular imprinted polymer solid phase extraction followed by LC-MS/MS.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.