C4879
α-Chymotrypsinogen A from bovine pancreas
essentially salt-free, lyophilized powder
Sinonimo/i:
chymotrypsin A zymogen
About This Item
Prodotti consigliati
Origine biologica
bovine pancreas
Livello qualitativo
Tipo
Type II
Forma fisica
essentially salt-free, lyophilized powder
Attività specifica
≥40 units/mg solid
PM
25,656 Da by calculation
Purificato mediante
6× crystallization
Solubilità
1 mM HCl: soluble 10 mg/mL, clear, colorless
N° accesso UniProt
Attività estranea
α-chymotrypsin ≤1 U/mg (prior to activation by trypsin)
Temperatura di conservazione
−20°C
Informazioni sul gene
cow ... CTRB1(618826)
Categorie correlate
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Definizione di unità
Altre note
Applicazioni
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
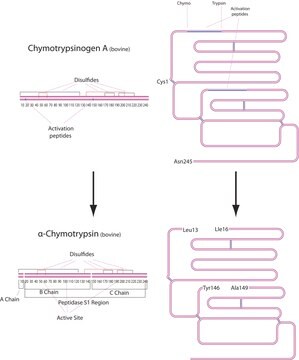
Analytical Enzyme Chymotrypsin: Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen.
Chromatograms
application for HPLCIl team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.