22620
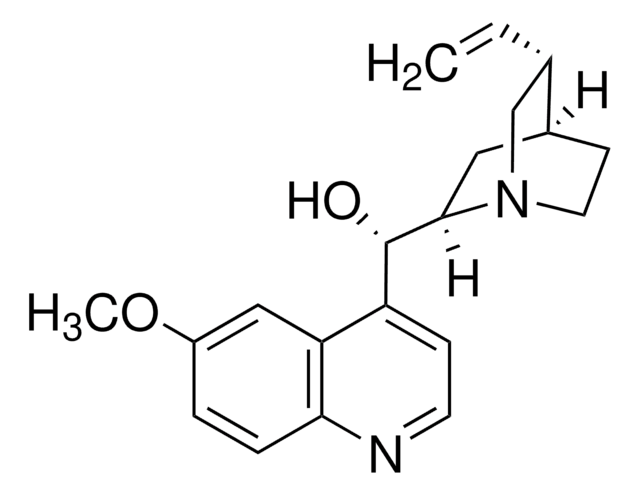
Quinine
suitable for fluorescence, anhydrous, ≥98.0% (dried material, NT)
Sinonimo/i:
6′-Methoxycinchonidine
About This Item
Prodotti consigliati
Saggio
≥98.0% (dried material, NT)
Stato
powder
Attività ottica
[α]20/D −126±5°, c = 1% in chloroform
Impurezze
≤5% dihydroquinine (HPLC)
Perdita
≤1% loss on drying, 110 °C
Punto di fusione
173-175 °C (lit.)
Solubilità
H2O: soluble
Fluorescenza
λex 347 nm; λem 448 nm in 0.5 M sulfuric acid
Compatibilità
suitable for fluorescence
Stringa SMILE
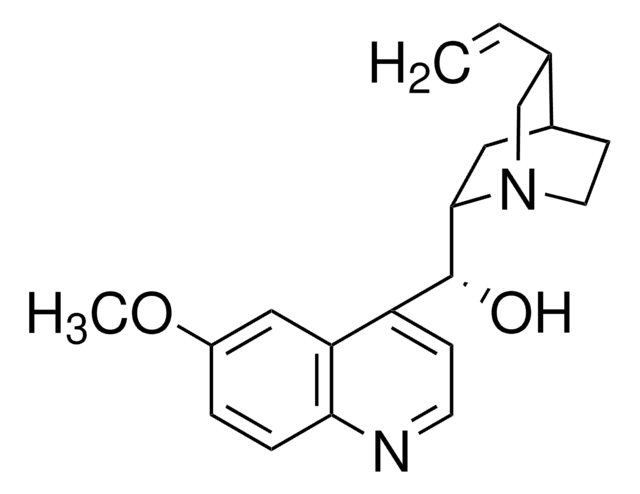
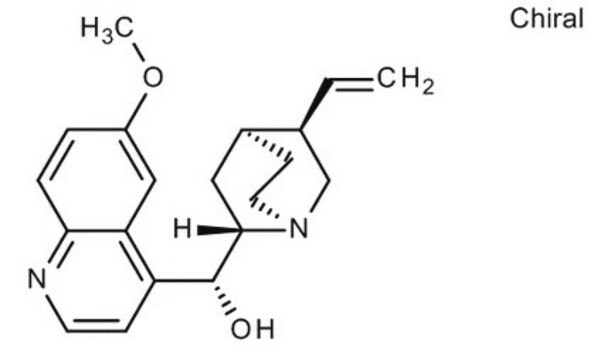
COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1
LOUPRKONTZGTKE-WZBLMQSHSA-N
Informazioni sul gene
human ... ABCB1(5243) , CYP2C9(1559) , CYP2D6(1565)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- To study its in vitro antimalarial activity in combination with omeprazole.
- To analyze its effect on viscosity and friction of saliva.
- As a test agent to study its impact on the accumulation of the fluorescent P-glycoprotein (Pgp) substrates in P-glycoprotein overexpressing breast cancer cells.
- To study its influence on the pyramidal cell intrinsic properties, extracellular potassium transients, and epileptiform activity in vitro.
- As a reference compound to identify alkaloids by phytochemical screening of Deianira erubescens, Strychnos pseudoquina and Remijia ferruginea plants.
Azioni biochim/fisiol
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Skin Sens. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.