732117
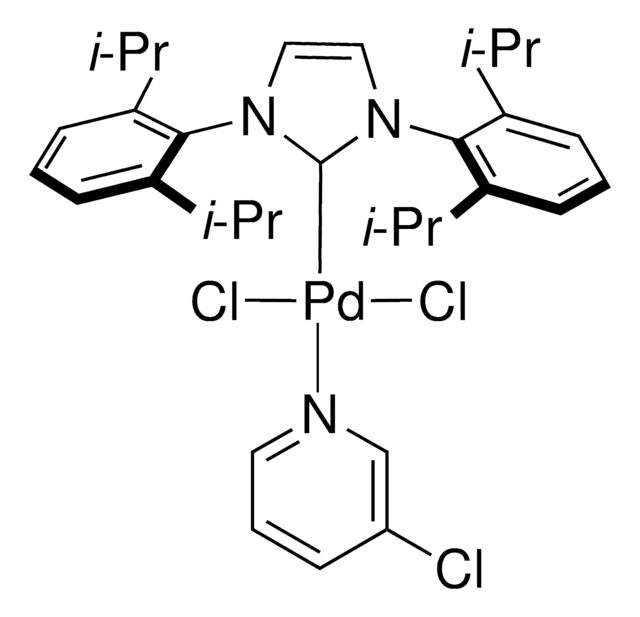
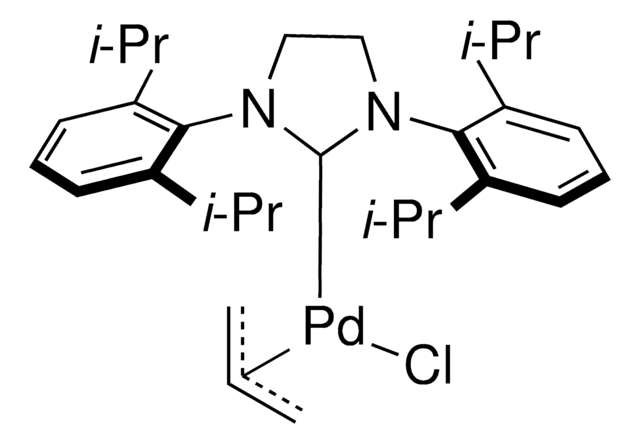
Pd-PEPPSI™-IPent catalyst
≥95%
Sinonimo/i:
Dichloro[1,3-bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II), [1,3-Bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)dichloropalladium(II), [1,3-Bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Stato
solid
Impiego in reazioni chimiche
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
Punto di fusione
195-201 °C
Temperatura di conservazione
−20°C
Stringa SMILE
Clc1cccnc1.CCC(CC)c2cccc(C(CC)CC)c2N3C=CN(c4c(cccc4C(CC)CC)C(CC)CC)\C3=[Pd](/Cl)Cl
InChI
1S/C35H52N2.C5H4ClN.2ClH.Pd/c1-9-26(10-2)30-19-17-20-31(27(11-3)12-4)34(30)36-23-24-37(25-36)35-32(28(13-5)14-6)21-18-22-33(35)29(15-7)16-8;6-5-2-1-3-7-4-5;;;/h17-24,26-29H,9-16H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
BCXSKTXOKALLAZ-UHFFFAOYSA-L
Descrizione generale
Applicazioni
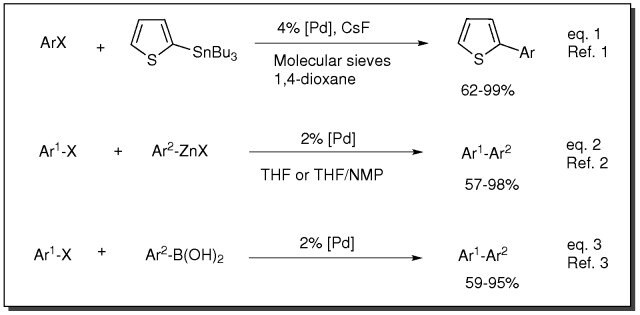
Cross-Coupling, Amination and Heck Transformation using PEPPSI Catalysts
- Catalyst for Stille coupling reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
For small scale and high throughput uses, product is also available as ChemBeads (928399)
Note legali
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
Professor Mike Organ at York University, along with co-workers Dr. Chris O’Brien and Dr. Eric Kantchev, have developed an palladium N-heterocyclic-carbene (NHC) catalyst system. They reacted PdCl2with a bulky NHC ligand, 2,6-diisopropylphenyllimidazolium chloride (IPr), and an α-donating 3-chloropyridine ligand for stability. The title complex, PEPPSI™, stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation. Sigma-Aldrich offesr gram-scale quantities of the PEPPSI™ catalyst in collaboration with the Organ research group.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
KitAlysis™ Suzuki-Miyaura Cross-Coupling Reaction Screening Kit
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 732117-1G | 4061832867168 |
| 732117-250MG | 4061832970226 |
| 732117-50G | |
| 732117-5G | 4061832970233 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.