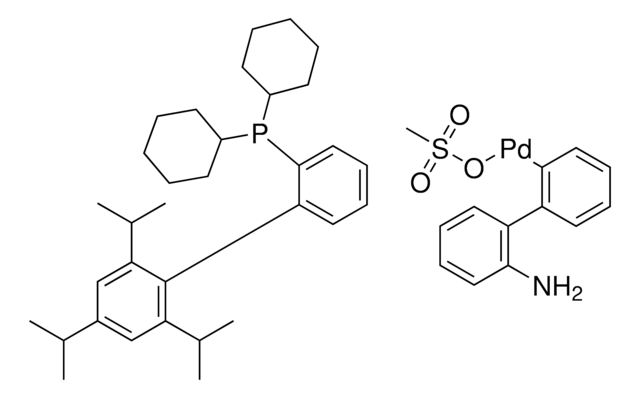
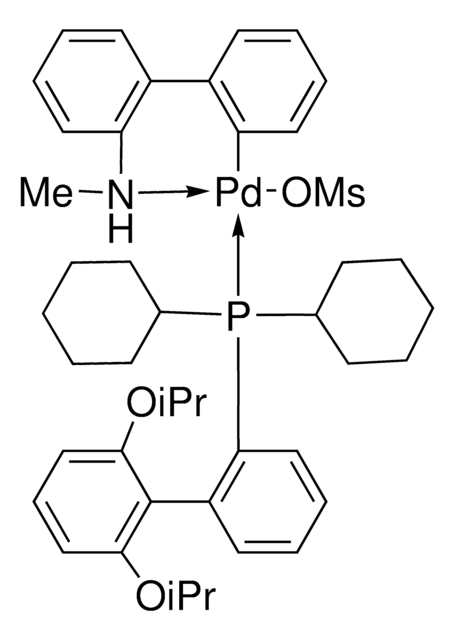
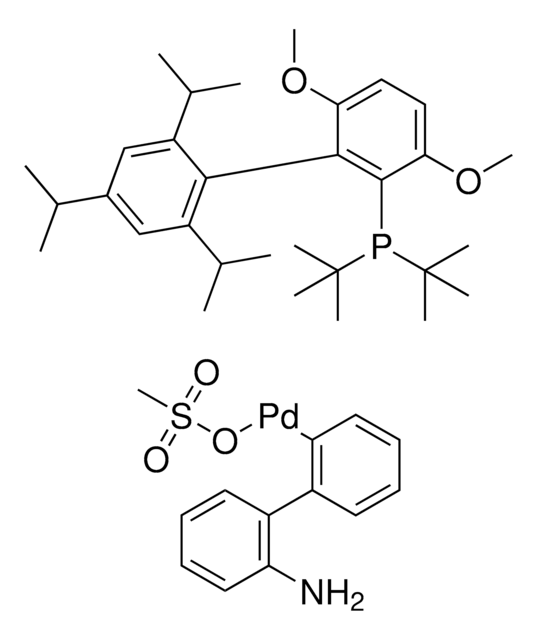
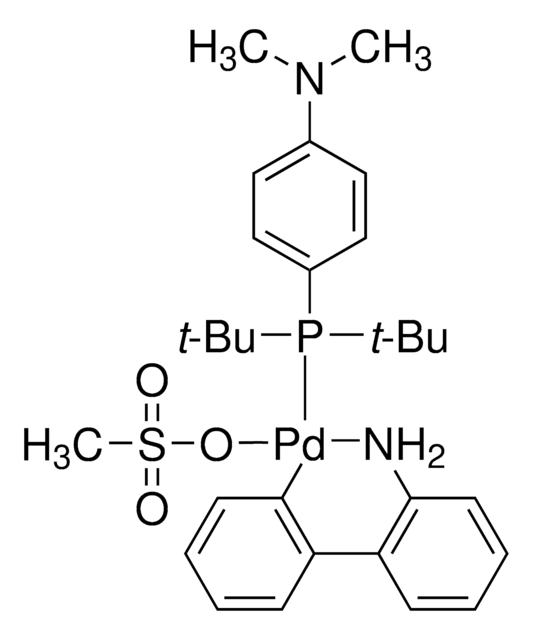
763403
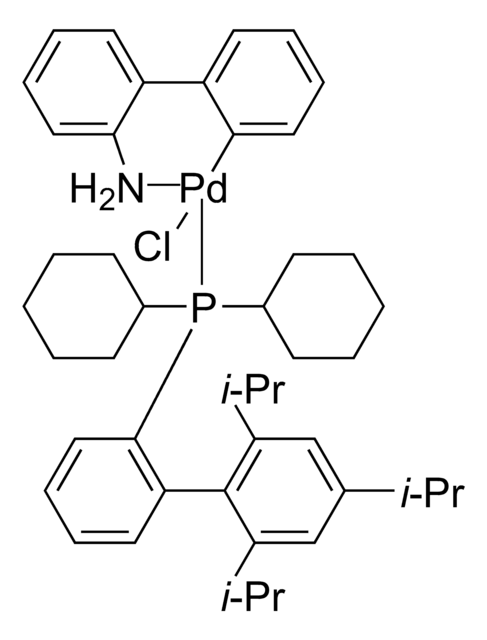
RuPhos Pd G3
98%
Sinonimo/i:
(2-Dicyclohexylphosphino-2′,6′-diisopropoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate, RuPhos-G3-Palladacycle, RuPhos-Pd-G3
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
solid
Caratteristiche
generation 3
Impiego in reazioni chimiche
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
Punto di fusione
188-196 °C (decomposition)
Gruppo funzionale
phosphine
Stringa SMILE
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CC(C)Oc3cccc(OC(C)C)c3-c4ccccc4P(C5CCCCC5)C6CCCCC6
InChI
1S/C30H43O2P.C12H10N.CH4O3S.Pd/c1-22(2)31-27-19-13-20-28(32-23(3)4)30(27)26-18-11-12-21-29(26)33(24-14-7-5-8-15-24)25-16-9-6-10-17-25;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h11-13,18-25H,5-10,14-17H2,1-4H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
AXZLIMCJMPNFFU-UHFFFAOYSA-M
Descrizione generale
Applicazioni
- Palladium-catalyzed Suzuki coupling of 5-p-toluenesulfonyltetrazoles with arylboronic acids to synthesize 1,5-disubstituted tetrazoles.
- Suzuki-Miyaura catalyst-transfer polycondensation (SCTP) of 3-alkylthiophenes in the presence of N-methylimidodiacetic (MIDA)-boronate monomers.
- Suzuki-Miyaura-cross-coupling of aminothiophenes.
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.