683434
(Dimethylphenylsilyl)boronic acid pinacol ester
95%
Sinonimo/i:
2-(Dimethylphenylsilyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, B-(dimethylphenylsilyl)pinacolborane
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C14H23BO2Si
Numero CAS:
Peso molecolare:
262.23
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
95%
Stato
liquid
Indice di rifrazione
n20/D 1.4946
Densità
0.962 g/mL at 25 °C
Stringa SMILE
CC1(C)OB(OC1(C)C)[Si](C)(C)c2ccccc2
InChI
1S/C14H23BO2Si/c1-13(2)14(3,4)17-15(16-13)18(5,6)12-10-8-7-9-11-12/h7-11H,1-6H3
ARMSAQNLTKGMGM-UHFFFAOYSA-N
Categorie correlate
Applicazioni
(Dimethylphenylsilyl)boronic acid pinacol ester (Suginome′s reagent) can be used as a reagent:
- For the selective addition of dimethylphenylsilanyl group to cyclic and acyclic unsaturated ketones, esters, acrylonitriles using a copper catalyst.
- In the synthesis of (Z)-4-boryl-1-silyl-2-alkene derivatives by stereoselective addition of silicon-boron bond to acyclic 1,3-dienes in presence of Ni catalyst.
- In the preparation of silyl-substituted butenoate and β-silyl-substituted acrylate derivatives from allenes and propiolate derivatives via hydrosilylation reactions using a copper catalyst.
- In the palladium-catalyzed asymmetric silaboration of allenes and alkanes to offered corresponding β-borylallylsilanes and 2-boryl-1-silylalkanes respectively.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes.
Lee K S and Hoveyda A H
Journal of the American Chemical Society, 132(9), 2898-2900 (2010)
Geminal Difunctionalization of Alkenylidene?Type Carbenoids by Using Interelement Compounds.
Hata T, et al.
Angewandte Chemie (International Edition in English), 40(4), 790-792 (2001)
Stereoselective 1, 4-silaboration of 1, 3-dienes catalyzed by nickel complexes
Suginome M, et al.
Organic Letters, 1(10), 1567-1569 (1999)
Platinum?Catalyzed Regioselective Silaboration of Alkenes.
Suginome M, et al.
Angewandte Chemie (International Edition in English), 36(22), 2516-2518 (1997)
Palladium-catalyzed asymmetric silaboration of allenes.
Ohmura T, et al.
Journal of the American Chemical Society, 128(42), 13682-13683 (2006)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
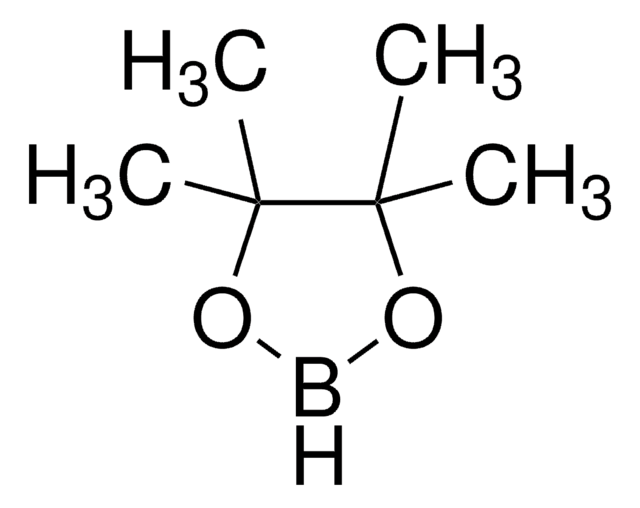
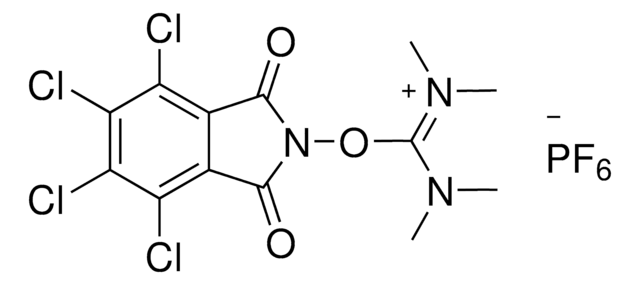
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
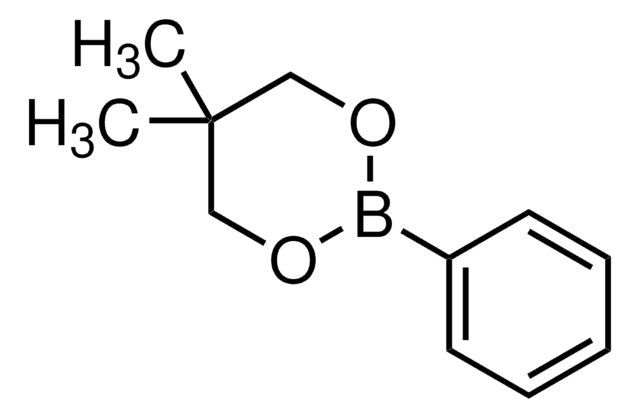

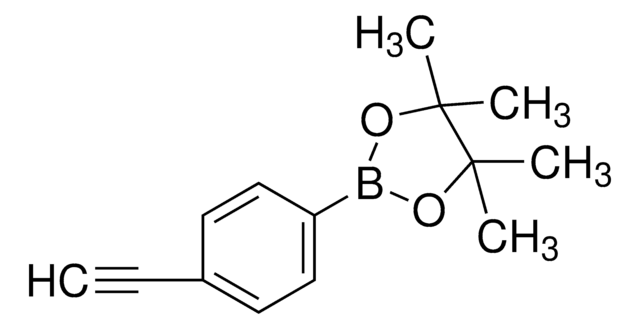
![Bis[(pinacolato)boryl]methane](/deepweb/assets/sigmaaldrich/product/structures/286/283/dcb13110-c536-4223-99e6-0dd505906b64/640/dcb13110-c536-4223-99e6-0dd505906b64.png)